Government Initiatives and Funding
Government initiatives aimed at improving healthcare access and quality are significantly influencing the point of-care-molecular-diagnostics market. In the UK, various funding programs and policies are being implemented to support the development and deployment of innovative diagnostic solutions. These initiatives are designed to enhance public health infrastructure and ensure that advanced diagnostic tools are accessible to a broader population. For instance, recent funding allocations have focused on expanding point-of-care testing capabilities in rural and underserved areas. This support is likely to drive market growth, as it encourages the adoption of molecular diagnostics in diverse healthcare settings, ultimately improving patient care and health outcomes.
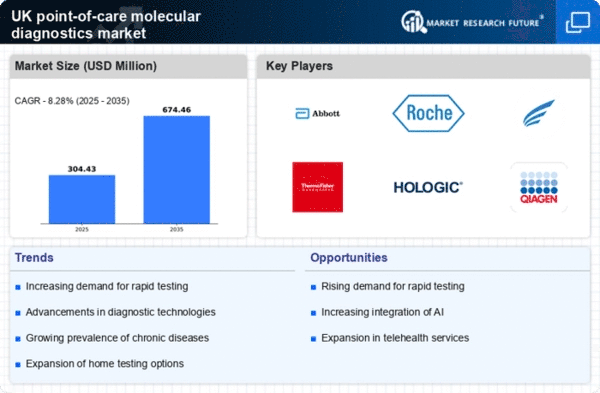
Rising Demand for Rapid Diagnostics
The increasing need for rapid diagnostic solutions is a key driver in the point of-care-molecular-diagnostics market. Healthcare providers are under pressure to deliver timely results, particularly in emergency settings. This demand is reflected in the market, which is projected to grow at a CAGR of approximately 10% over the next five years. The ability to obtain results within minutes rather than days enhances patient management and treatment outcomes. Furthermore, the shift towards outpatient care and home testing is likely to bolster the adoption of point-of-care testing devices, as they provide immediate results that facilitate quicker clinical decisions. As a result, the point of-care-molecular-diagnostics market is expected to expand significantly, driven by the necessity for speed in diagnostic processes.
Growing Focus on Preventive Healthcare
The increasing emphasis on preventive healthcare is emerging as a significant driver for the point of-care-molecular-diagnostics market. As healthcare systems shift towards proactive management of health, the demand for early detection and monitoring of diseases is rising. Point-of-care molecular diagnostics offer the advantage of identifying health issues before they escalate, which aligns with the preventive healthcare model. This trend is expected to propel market growth, with projections indicating that preventive diagnostics could represent a substantial portion of the market by 2027. The ability to conduct tests in various settings, including homes and community clinics, further supports this shift, making the point-of-care-molecular-diagnostics market a critical component of future healthcare strategies.
Technological Integration in Healthcare
The integration of advanced technologies into healthcare systems is driving the evolution of the point of-care-molecular-diagnostics market. Innovations such as artificial intelligence, machine learning, and mobile health applications are enhancing the capabilities of diagnostic devices. These technologies enable more accurate and efficient testing, which is crucial for effective patient management. The market is witnessing a surge in the development of smart diagnostic tools that can provide real-time data analysis and connectivity to electronic health records. This trend is expected to contribute to a market growth rate of around 12% annually, as healthcare providers increasingly adopt these technologies to improve patient outcomes and streamline operations.
Increased Prevalence of Infectious Diseases
The rising incidence of infectious diseases in the UK is a substantial driver for the point of-care-molecular-diagnostics market. With the growing burden of diseases such as respiratory infections, sexually transmitted infections, and gastrointestinal diseases, there is an urgent need for effective diagnostic tools. The market is responding to this need, with estimates suggesting that the infectious disease segment could account for over 40% of the total market share by 2026. The ability to quickly identify pathogens at the point of care not only aids in timely treatment but also helps in controlling outbreaks. Consequently, The point-of-care molecular diagnostics market is likely to see increased investment and innovation aimed at addressing these public health challenges.

















Leave a Comment