Supportive Government Initiatives
Supportive government initiatives play a crucial role in the growth of the point of-care-molecular-diagnostics market in South Korea. The government has been actively promoting the development and adoption of innovative diagnostic technologies through funding and regulatory support. Initiatives aimed at enhancing healthcare infrastructure and accessibility are likely to encourage the integration of point-of-care testing in various healthcare settings. Furthermore, public health campaigns emphasizing the importance of early diagnosis and treatment are expected to drive demand for molecular diagnostics. This supportive environment fosters innovation and investment, positioning the market for sustained growth in the coming years.
Rising Demand for Rapid Diagnostics
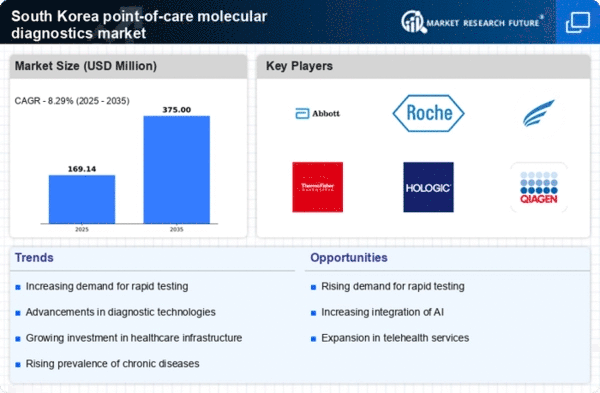
The increasing need for rapid diagnostic solutions is a key driver in the point of-care-molecular-diagnostics market. In South Korea, healthcare providers are increasingly prioritizing quick and accurate testing methods to enhance patient outcomes. This trend is particularly evident in emergency departments and outpatient settings, where timely diagnosis can significantly impact treatment decisions. The market is projected to grow at a CAGR of approximately 10% over the next few years, driven by the demand for devices that can deliver results within minutes. As healthcare systems strive for efficiency, the adoption of point-of-care molecular diagnostics is likely to expand, reflecting a shift towards more immediate healthcare solutions.
Integration of Advanced Technologies
The integration of advanced technologies such as artificial intelligence (AI) and machine learning into the point of-care-molecular-diagnostics market is transforming the landscape of medical testing in South Korea. These technologies enhance the accuracy and efficiency of diagnostic devices, allowing for better data analysis and interpretation. For instance, AI algorithms can assist in identifying patterns in test results, leading to more precise diagnoses. The market is witnessing a surge in investments aimed at developing innovative diagnostic tools that leverage these technologies. This trend not only improves patient care but also positions South Korea as a leader in the adoption of cutting-edge healthcare solutions.
Growing Prevalence of Infectious Diseases
The rising prevalence of infectious diseases in South Korea is a significant driver for the point of-care-molecular-diagnostics market. With an increase in the incidence of conditions such as respiratory infections and sexually transmitted diseases, there is a heightened demand for rapid and accurate diagnostic tests. The ability to quickly identify pathogens is crucial for effective treatment and containment of outbreaks. As a result, healthcare facilities are increasingly investing in point-of-care molecular diagnostics to enhance their diagnostic capabilities. This trend is expected to contribute to a robust growth trajectory for the market, as healthcare providers seek to improve their response to infectious disease challenges.
Increasing Focus on Personalized Medicine
The increasing focus on personalized medicine is emerging as a significant driver in the point of-care-molecular-diagnostics market. In South Korea, there is a growing recognition of the need for tailored treatment approaches based on individual patient profiles. Molecular diagnostics enable healthcare providers to identify specific biomarkers and genetic information, facilitating more effective treatment plans. This trend aligns with the broader shift towards precision medicine, where therapies are customized to the unique characteristics of each patient. As healthcare professionals seek to enhance treatment efficacy, the demand for point-of-care molecular diagnostics is likely to rise, reflecting a commitment to improving patient outcomes.


















Leave a Comment