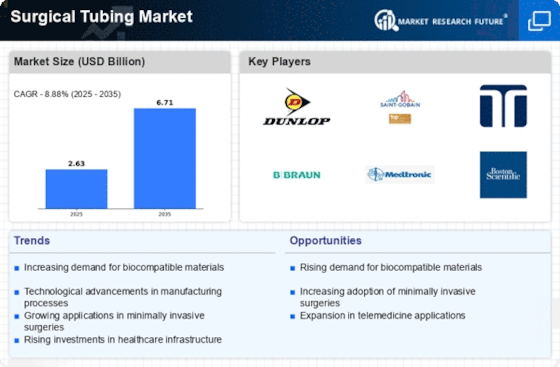
Market Analysis
In-depth Analysis of Surgical Tubing Market Industry Landscape
The Stem Cell Reconstructive Market is experiencing noteworthy growth, fueled by advancements in regenerative medicine and the use of stem cells for reconstructive therapies. Stem cell-based interventions have emerged as a promising approach to address a wide range of medical conditions and injuries. Stem cells possess unique regenerative properties, making them valuable for reconstructive purposes. These cells can differentiate into various cell types, promoting tissue repair and regeneration in damaged or diseased areas of the body. Stem cell reconstructive market is driven by the orthopedic segment. Stem cells are used in orthopedic treatment and helps bone cartilage, and soft tissue to repair the damaged section by regeneration its is a new approach for osteoarthritis as well treats muscle injury. The application of tracts based on stem cell reconstructive therapies is highly in plastic and reconstructive surgery. Facelift, breast reconstruction and many other procedures may benefit from the use of stem cells in terms of improvement for speedy healing favorable tissue quality along with better cosmetic results. Nationally, stem cells are critical to cardiovascular restoration. Techniques that employ stem cell therapies are intended to reduce the damage incurred on heart tissues, presenting new treatment opportunities for diseases of the heart and general improvement of cardiac function. Some promise is seriously being shown by stem cell-based strategies in dealing with microscopic structure and spinal line lesions. Specialists try to understand the ability of stem cells repair neural tissues damaged due for example, Parkinson’s disease and spinal cord injury which restores function in patients. Recent innovations in stem cell technologies facilitate successful reconstruction. New approaches to isolation, culture and alteration of stem cells increase their possibilities in treatments planting new horizons. Stimulated by a dynamic environment of clinical trials and research programs for stem cells, the U.S. reconstructive market has been cordoned The scientific communities’ researches aimed at discovery of new areas for applications and increasing the numbers on evidence behind stem cell therapies launch stimulate market expansion. The regulatory sphere within which stem cell therapies are operating changes. Monitoring bodies are in the process of devising policies to ensure that these intervention s offer safety and efficiency while providing a moderation proportionality on its usage. The patient-driven healthcare model becomes more prominent, and the changes to that end structure stem cell reconstructive therapies. Stem cell therapy in reconstructive medicine is increasingly characterized by personalized treatment plans adjusted to individual patient needs. Standardization is an ongoing challenge; the scalability of such therapies over long periods of time has yet to be determined. The efforts to resolve such challenges are necessary for the formation of long-lasting and trusted approaches in it. Strategic affiliations among research centers, biotechnology companies and healthcare providers are the crux of commercializing stem cell reconstructive therapies. Such partnerships allow to convert scientific findings into implementable and available treatments. The impact of the U.S. Stem Cell Reconstructive Market extends beyond national borders, contributing to global advancements in regenerative medicine. Collaborations and knowledge sharing on an international scale enhance the collective understanding of stem cell applications.
















Leave a Comment