Rising Incidence of Chronic Diseases
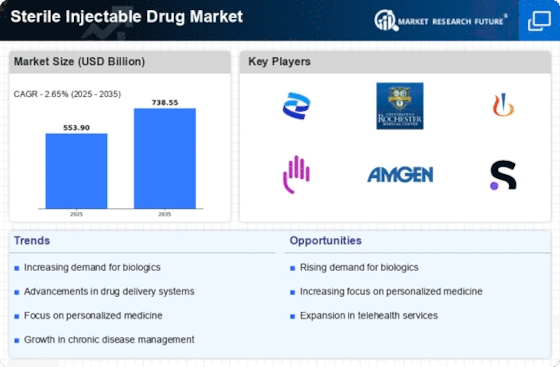
The Sterile Injectable Drug Market is experiencing growth due to the increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders. According to recent data, chronic diseases account for approximately 70% of all deaths worldwide, necessitating effective treatment options. Injectable drugs are often preferred for their rapid onset of action and higher bioavailability, making them essential in managing these conditions. As healthcare systems strive to improve patient outcomes, the demand for sterile injectables is likely to rise. This trend is further supported by the growing aging population, which is more susceptible to chronic illnesses. Consequently, pharmaceutical companies are focusing on developing innovative sterile injectable formulations to cater to this expanding patient demographic.
Advancements in Drug Delivery Systems
Innovations in drug delivery systems are significantly influencing the Sterile Injectable Drug Market. The development of advanced delivery mechanisms, such as microneedles and smart injectors, enhances the efficacy and safety of injectable medications. These technologies not only improve patient compliance but also reduce the risk of needle-stick injuries, which is a critical concern in healthcare settings. Market data indicates that the global market for drug delivery systems is projected to reach USD 1.5 trillion by 2025, with a substantial portion attributed to sterile injectables. As these technologies evolve, they are expected to facilitate the administration of complex biologics and biosimilars, further driving the demand for sterile injectables in various therapeutic areas.
Growing Demand for Home Healthcare Solutions
The Sterile Injectable Drug Market is benefiting from the rising demand for home healthcare solutions. As patients increasingly prefer receiving treatments in the comfort of their homes, the need for sterile injectable drugs that can be administered outside traditional healthcare settings is growing. This trend is particularly evident in the management of chronic diseases, where self-administration of injectable medications can enhance patient adherence and satisfaction. Market Research Future indicates that the home healthcare market is expected to reach USD 300 billion by 2025, with a significant portion attributed to injectable therapies. Consequently, pharmaceutical companies are focusing on developing user-friendly sterile injectables that cater to this emerging market, thereby driving growth in the sterile injectable drug sector.
Increasing Focus on Biologics and Biosimilars
The Sterile Injectable Drug Market is witnessing a shift towards biologics and biosimilars, which are increasingly being recognized for their therapeutic potential. Biologics, derived from living organisms, often require sterile injectable formulations for administration. The market for biologics is projected to grow significantly, with estimates suggesting it could reach USD 500 billion by 2025. This growth is driven by the rising incidence of autoimmune diseases and cancer, where biologics have shown promising results. Additionally, the introduction of biosimilars is expected to enhance market competition, leading to reduced costs and improved access to these therapies. As healthcare providers seek effective treatment options, the demand for sterile injectables in this segment is likely to expand.
Regulatory Support for Injectable Drug Development
Regulatory bodies are increasingly supporting the development of sterile injectables, which is positively impacting the Sterile Injectable Drug Market. Initiatives aimed at streamlining the approval process for injectable drugs are being implemented, allowing for faster market entry of new therapies. For instance, the FDA has introduced guidelines that facilitate the development of complex generics and biosimilars, which often require sterile injectable formulations. This regulatory evolution is crucial in addressing the unmet medical needs of patients and ensuring timely access to innovative therapies. As a result, pharmaceutical companies are more inclined to invest in the development of sterile injectables, anticipating a favorable regulatory environment that encourages innovation and expedites the approval process.