Market Trends
Key Emerging Trends in the Spindle Cell Sarcoma Treatment Market
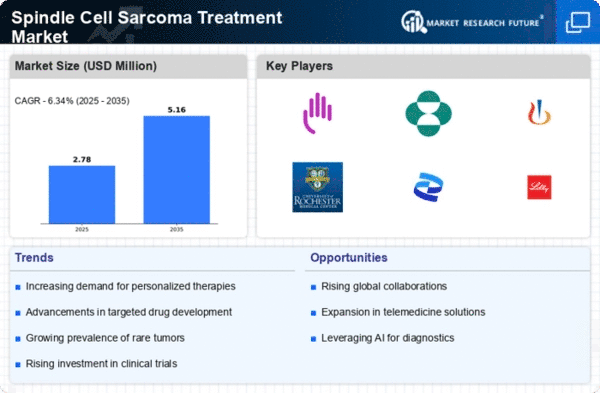
The market for spindle cell sarcoma treatment, a rare type of soft tissue sarcoma characterized by spindle-shaped cells, is witnessing a growing incidence globally. Factors such as improved diagnostic techniques, increased awareness among healthcare professionals, and advances in molecular pathology contribute to the rising identification of spindle cell sarcoma cases. The group of the sarcomas of the spindle cells is the name which is given to the complex of mesenchymal tumors arising from various troubling sites, such as leiomyosarcoma, Gastrointestinal Stromal Tumor (GIST) and spindle cell carcinoma. The classification of histological subtype is of paramount importance, especially for determining the treatment regimen and patient prognosis. Therefore, histopathological diagnosis carries an enormous amount of weight, too, in the spindle cell sarcoma management. Maximizing surgical resection within anal cancer negative margins along with preserving of functional and vital structures represents the primary mode of therapy for locally advanced spindle cell sarcomas. Development of minimally invasive approaches, including limb-sparing surgery and microsurgical reconstruction, is a commonplace and leads to the patients' overall better surgical result and quality of life. An adjuvant radiotherapy is indicated to that the risk for local recurrence could be reduced and local control could be achieved. Radiation therapy can come prior to, during or after the surgery, or it can be a definitive treatment for the inoperable or invincible tumours, with the modernization in radiotherapy techniques leading to precise targeting and lessen negative impacts. Chemotherapy treatments are effective in combating the systemic progression of advanced or metastatic phase of the spindle cell sarcomas attacking both high-grade or unresectable tumors. Chemotherapeutics such as anthracyclines, ifosfamide, and basically all taxanes are among the tinctures that can be used in combination protocol to induce tumor regression and improve survival outcomes of patients. Nevertheless, within different patients the response to treatment can vary.
Precision medicine approaches in spindle cell sarcoma treatment involve the molecular profiling of tumors to identify actionable genetic alterations and guide personalized treatment strategies. Molecular testing, including next-generation sequencing (NGS) and gene expression profiling, enables oncologists to tailor treatment regimens based on tumor-specific molecular characteristics, optimizing therapeutic efficacy and minimizing treatment-related toxicity. Intensifying competition among pharmaceutical companies, medical device manufacturers, and healthcare providers influences market dynamics in the spindle cell sarcoma treatment market. Companies differentiate their products and services through technological innovations, clinical efficacy, and pricing strategies to gain a competitive edge and address the evolving needs of patients and healthcare providers.

















Leave a Comment