Market Analysis
In-depth Analysis of Spindle Cell Sarcoma Treatment Market Industry Landscape
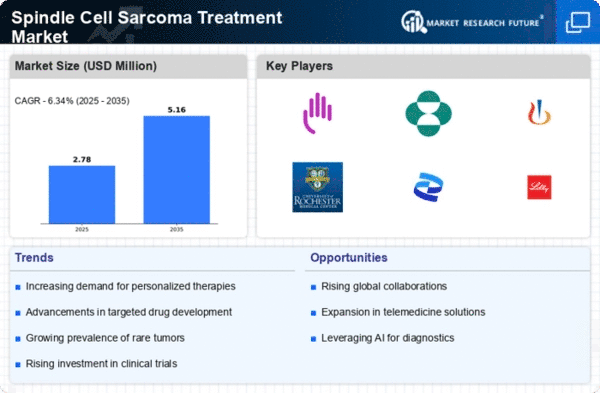
Spindle cell sarcoma is a rare type of soft tissue sarcoma characterized by spindle-shaped cells in tumors that can occur in various parts of the body, including the limbs, trunk, and abdomen. Due to its rarity, the market for spindle cell sarcoma treatments is relatively niche compared to more common cancers, posing unique challenges and opportunities for stakeholders. The prevalent market dynamics of cell spindling sarcoma treatment are determined by limited available effective treatment options. Surgery to take away the tumor is usually the main treatment approach, though that location and this whole extent of the tumor may serve as a challenging barrier to remove it completely. Another kind of cancer that is more widespread and one of the most challenging to treat is spindle cell sarcoma. This is due to the fact that chemotherapy and radiation therapy are successful in treating it having low efficiency, which leads to the necessity of other therapies. Research programs have been directed to the eventuality of precise therapy that can actually bring tumor cells to an end. The goal of these therapies is to identify mutations at the specific epigenetic level or motivated molecular pathways in which the cancer develops and spreads. Individual scope of targeting therapies allows to administer more precise and effective treatment which consequently provides the best results for patients with spindle cell sarcoma disease. This regulatory environment that circles around the treatment of spindle cell sarcoma with regulatory authorities is doing their best in providing assurance of safety, effectiveness, and affordability. Regulation is particularly complex in this field due to the need to address new medication approvals mechanisms, guidelines for clinical practices, as well as protocols for such patients as those with spindle cell sarcoma. The way for companies wanting to access the spindle cell sarcoma treatment market is the entrance barriers, whose most important elements are: regulatory obstacles, competition from players already on the market and clinical evidence showing safety and effectiveness of novel therapies. Sensing and overcoming these barriers is no easy task and demands a deep understanding of the working force of the laws and commercial environment.
Despite the challenges associated with spindle cell sarcoma treatment, the market presents opportunities for expansion and innovation. Advances in targeted therapies, immunotherapies, and personalized medicine hold promise for improving outcomes and quality of life for patients with spindle cell sarcoma. Collaboration among stakeholders, including healthcare providers, researchers, pharmaceutical companies, and patient advocacy groups, is essential for driving progress in the field. Looking ahead, the market dynamics of spindle cell sarcoma treatment are expected to evolve with advancements in research, technology, and healthcare delivery. Efforts to identify targeted therapies, optimize treatment approaches, and improve patient access to innovative treatments will continue to shape the market landscape, offering hope for patients with spindle cell sarcoma.

















Leave a Comment