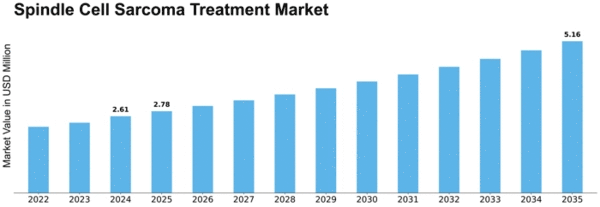
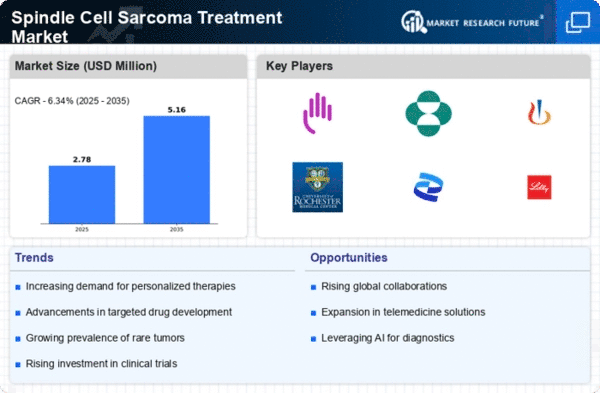
Spindle Cell Sarcoma Treatment Size
Spindle Cell Sarcoma Treatment Market Growth Projections and Opportunities
Spindle cell sarcoma is a rare type of cancer, affecting a small percentage of the population, which influences market dynamics by limiting the patient pool and affecting the availability of specialized treatment options and research funding. The development of new technologies through MRI , CT and PET scans has increased the efficiency and risen the accuracy of diagnostics of spindle cell sarcoma. This has affected the markets dynamics by encouraging early detection and treatment planning. Genetically transmitted carcinogenic [substances] and the environment are the predisposing factors of the Affections of spindle cells while market demand is driven by the exploration of personalized treatment approaches targeting genetic mutations and environmental risk factors. Schemes for research and development funding which extends to the private sector, public universities, and government agencies in turn increases cancer treatment innovation in spindle cell sarcoma, thereby boosting the pipeline of potential medicines and the market landscape. Tightening guidelines for pharmaceuticals and medical devices implementation will weigh upon R&D companies which develops products and services in spindle cell sarcoma treatment, leading to potential delays in product and service availability, high costs due to expensive needed to obtain approval, and increased competition. Alterations in a health insurance market for cancer treatment can affect the accessibility and affordability for patients and taken to the account of the patients’ health during the considering sarcoma management. Patient participation in clinical trials directed toward spindle cell sarcomas has the potential to transform the market dynamics as it broadens the range of available treatment options for patients, motivates new research, and creates a previous access to the treatment alternatives not available on the market yet. Increasingly, patient support groups advocate and promote through these activities no matter how small, gain public awareness, drive research funding, and affect healthcare policies. The end result is that what is available for the individuals with spindle cell sarcoma in terms of care and support undergoes improvement or change.
Increasing awareness campaigns and patient education initiatives raise awareness about the signs, symptoms, and treatment options for spindle cell sarcoma, driving market growth by facilitating early diagnosis and treatment-seeking behavior among patients. Increasing competition among pharmaceutical companies, medical device manufacturers, and healthcare providers in the spindle cell sarcoma treatment market drives innovation, affordability, and accessibility of treatment options, shaping the competitive landscape of the market.

















Leave a Comment