Increasing Healthcare Expenditure
The non invasive-prenatal-testing market is benefiting from the rising healthcare expenditure in South Korea. As the government and private sectors invest more in healthcare services, the accessibility and affordability of prenatal testing are improving. In 2025, healthcare expenditure in South Korea is expected to reach approximately 9% of GDP, reflecting a commitment to enhancing maternal and child health services. This increase in funding allows for the development and distribution of advanced non invasive testing options, making them more widely available to expectant parents. Furthermore, as healthcare providers expand their offerings, the non invasive-prenatal-testing market is likely to see a surge in adoption rates. The correlation between increased healthcare spending and the growth of the non invasive-prenatal-testing market suggests a promising future for prenatal diagnostics.
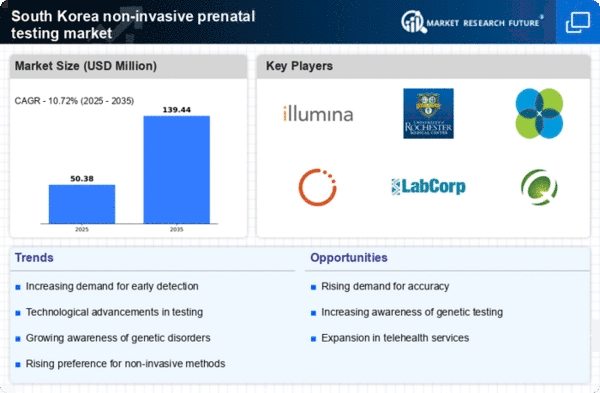
Rising Demand for Early Detection
The non invasive-prenatal-testing market in South Korea is experiencing a notable increase in demand for early detection of genetic disorders. This trend is largely driven by the growing awareness among expectant parents regarding the benefits of early diagnosis. In 2025, it is estimated that approximately 30% of pregnant women in South Korea opt for non invasive prenatal testing to identify potential genetic abnormalities. This shift towards proactive healthcare is indicative of a broader societal trend prioritizing maternal and fetal health. As a result, healthcare providers are increasingly integrating non invasive testing into routine prenatal care, thereby expanding the market's reach and accessibility. The rising demand for early detection is expected to propel the market forward, as more families seek to make informed decisions about their pregnancies.
Technological Innovations in Testing
Technological advancements play a crucial role in shaping the non invasive-prenatal-testing market. In South Korea, innovations such as next-generation sequencing (NGS) have significantly enhanced the accuracy and efficiency of prenatal tests. These technologies allow for the analysis of cell-free fetal DNA, providing reliable results with minimal risk to the mother and fetus. As of 2025, the market is projected to grow at a CAGR of 15%, driven by these technological innovations. The introduction of user-friendly testing kits and improved data analysis software further supports this growth. Healthcare providers are increasingly adopting these advanced technologies, which not only improve diagnostic capabilities but also enhance patient experience. Consequently, the integration of cutting-edge technology is likely to remain a key driver in the non invasive-prenatal-testing market.
Regulatory Support for Testing Standards
Regulatory support is emerging as a vital driver for the non invasive-prenatal-testing market in South Korea. The government is actively establishing guidelines and standards to ensure the safety and efficacy of prenatal testing methods. In 2025, regulatory bodies are expected to implement more stringent quality control measures, which will enhance consumer confidence in non invasive testing. This regulatory framework not only protects patients but also encourages innovation within the industry. As companies comply with these regulations, they are likely to invest in research and development, leading to the introduction of more advanced testing solutions. The establishment of a robust regulatory environment is anticipated to foster growth in the non invasive-prenatal-testing market, as it aligns with the increasing demand for reliable and safe prenatal diagnostics.
Cultural Shift Towards Preventive Healthcare
A cultural shift towards preventive healthcare is influencing the non invasive-prenatal-testing market in South Korea. As societal attitudes evolve, there is a growing emphasis on the importance of early intervention and preventive measures in healthcare. This shift is particularly evident among younger generations, who are more inclined to seek out non invasive prenatal testing as a means of ensuring the health of their unborn children. In 2025, it is anticipated that around 40% of first-time mothers will opt for non invasive testing, reflecting this cultural change. The increasing acceptance of preventive healthcare practices is likely to drive market growth, as more individuals recognize the value of early detection in managing potential health risks. This cultural transformation may also encourage healthcare providers to promote non invasive testing as a standard part of prenatal care.