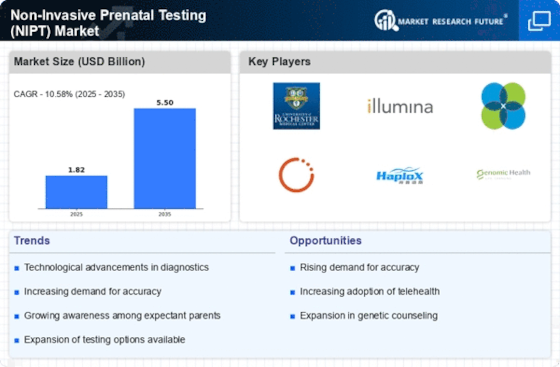
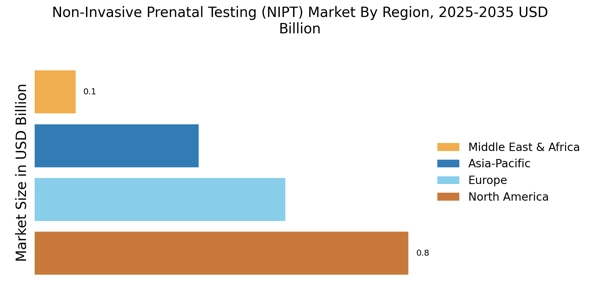
Increased Focus on Maternal Health
The heightened emphasis on maternal health and wellness is a crucial driver for the Non-Invasive Prenatal Testing (NIPT) Market. As healthcare systems worldwide prioritize maternal care, there is a growing recognition of the importance of early prenatal screening. This focus is reflected in various initiatives aimed at improving maternal health outcomes, which often include the promotion of NIPT as a standard practice. Data suggests that countries with robust maternal health programs are witnessing a rise in NIPT adoption rates, as healthcare providers advocate for its benefits. The increasing investment in maternal health initiatives is likely to further bolster the NIPT market, as more women gain access to these essential testing services.
Rising Incidence of Genetic Disorders
The increasing prevalence of genetic disorders among newborns is a pivotal driver for the Non-Invasive Prenatal Testing (NIPT) Market. As more parents seek to understand potential genetic risks, the demand for NIPT has surged. Reports indicate that conditions such as Down syndrome and other chromosomal abnormalities are becoming more recognized, prompting expectant parents to opt for early screening. This trend is further supported by advancements in genetic research, which have made it easier to identify at-risk pregnancies. Consequently, healthcare providers are increasingly recommending NIPT as a reliable option for early detection, thereby expanding its market reach. The rising incidence of genetic disorders is likely to continue influencing the NIPT market, as awareness grows and testing becomes more accessible.
Regulatory Endorsements and Guidelines
Regulatory support and endorsements play a vital role in shaping the Non-Invasive Prenatal Testing (NIPT) Market. As health authorities recognize the benefits of NIPT, they are establishing guidelines that promote its use in clinical practice. These endorsements not only enhance the credibility of NIPT but also encourage healthcare providers to adopt these testing methods. In many regions, regulatory bodies are actively working to ensure that NIPT is included in standard prenatal care protocols, which is likely to increase its accessibility. The establishment of clear guidelines and recommendations is expected to drive market growth, as more healthcare professionals become aware of the advantages of NIPT and its role in improving prenatal care.
Changing Demographics and Delayed Parenthood
Shifts in demographics, particularly the trend of delayed parenthood, are influencing the Non-Invasive Prenatal Testing (NIPT) Market. As more individuals choose to start families later in life, the associated risks of chromosomal abnormalities increase. This demographic shift has led to a greater demand for prenatal testing options that can provide early insights into potential genetic issues. Consequently, healthcare providers are increasingly recommending NIPT as a proactive measure for older expectant mothers. The market data indicates that women over the age of 35 are among the fastest-growing segments opting for NIPT, thereby driving market expansion. This trend is expected to continue, as societal norms evolve and the age of first-time parents rises.
Technological Innovations in Testing Methods
Technological advancements in testing methodologies are significantly propelling the Non-Invasive Prenatal Testing (NIPT) Market. Innovations such as next-generation sequencing (NGS) have enhanced the accuracy and efficiency of prenatal tests, allowing for the detection of fetal DNA in maternal blood with remarkable precision. These advancements not only improve the reliability of results but also reduce the time required for testing, making it more appealing to expectant parents. Furthermore, the integration of artificial intelligence in data analysis is expected to streamline processes and enhance predictive capabilities. As technology continues to evolve, it is anticipated that the NIPT market will experience substantial growth, driven by the demand for more sophisticated and user-friendly testing options.