Growing Awareness and Education
The increasing awareness and education surrounding psoriasis and its treatment options are vital drivers for the Psoriasis Biosimilar Market. Healthcare professionals and patients are becoming more informed about the benefits of biosimilars, which enhances their acceptance and utilization. Educational initiatives aimed at both providers and patients are crucial in dispelling misconceptions about biosimilars, thereby fostering a more favorable perception. As awareness grows, patients are more likely to inquire about biosimilar options, leading to higher adoption rates. Furthermore, healthcare providers are increasingly recognizing the potential of biosimilars to offer effective treatment alternatives. This shift in understanding is expected to contribute positively to the growth trajectory of the Psoriasis Biosimilar Market.
Cost-Effectiveness of Biosimilars
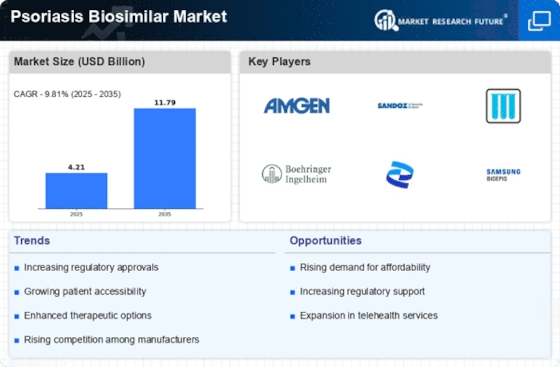
Cost considerations play a crucial role in the expansion of the Psoriasis Biosimilar Market. Biosimilars are generally priced lower than their reference biologics, making them an attractive option for healthcare providers and patients alike. The potential for significant cost savings can lead to increased adoption rates among patients who may have previously been unable to afford biologic therapies. According to recent data, the introduction of biosimilars has the potential to reduce treatment costs by up to 30-40%, thereby enhancing accessibility for a broader patient demographic. This economic advantage is particularly relevant in healthcare systems facing budget constraints, where the demand for affordable treatment options is paramount. As a result, the cost-effectiveness of biosimilars is likely to drive their uptake in the Psoriasis Biosimilar Market.
Increasing Prevalence of Psoriasis
The rising incidence of psoriasis is a pivotal driver for the Psoriasis Biosimilar Market. Recent estimates indicate that psoriasis affects approximately 2-3% of the population in various regions, leading to a growing demand for effective treatment options. As more individuals seek management solutions for this chronic condition, the market for biosimilars is likely to expand. The increasing awareness of psoriasis and its impact on quality of life further fuels this demand. Consequently, pharmaceutical companies are focusing on developing biosimilars that offer similar efficacy to existing biologics but at a lower cost. This trend not only enhances patient access to treatment but also stimulates competition among manufacturers, potentially leading to more innovative solutions in the Psoriasis Biosimilar Market.
Advancements in Regulatory Frameworks
The evolving regulatory landscape is another significant driver for the Psoriasis Biosimilar Market. Regulatory agencies are increasingly establishing clear pathways for the approval of biosimilars, which facilitates their entry into the market. This trend is evident in various regions, where streamlined processes and guidelines are being implemented to ensure the safety and efficacy of biosimilars. For instance, the introduction of abbreviated pathways for biosimilar approval has accelerated the development timeline, allowing manufacturers to bring their products to market more swiftly. As regulatory bodies continue to support the biosimilar sector, the confidence of healthcare providers and patients in these products is likely to grow, further propelling the expansion of the Psoriasis Biosimilar Market.
Technological Innovations in Biologics
Technological advancements in the development of biologics are significantly influencing the Psoriasis Biosimilar Market. Innovations in manufacturing processes, such as improved cell culture techniques and purification methods, are enhancing the quality and consistency of biosimilars. These advancements not only ensure that biosimilars meet stringent regulatory standards but also contribute to their overall efficacy and safety profiles. As manufacturers adopt cutting-edge technologies, the production of biosimilars becomes more efficient, potentially lowering costs and increasing availability. This technological evolution is likely to attract more players into the market, fostering competition and driving further innovation. Consequently, the impact of technological innovations is expected to be a key factor in shaping the future of the Psoriasis Biosimilar Market.