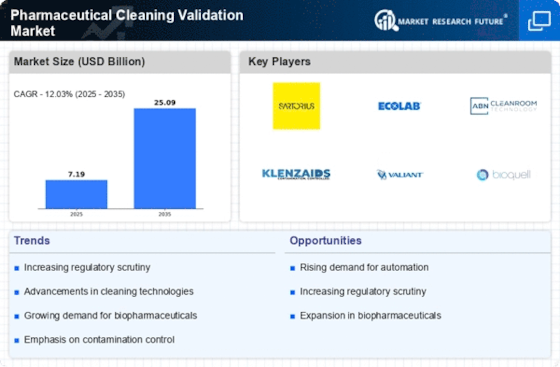
Stringent Regulatory Requirements
Regulatory bodies are imposing increasingly stringent requirements for cleaning validation in the Pharmaceutical Cleaning Validation Market. These regulations are designed to ensure that pharmaceutical products are manufactured in a safe and compliant manner, minimizing the risk of contamination. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established comprehensive guidelines that necessitate rigorous cleaning validation processes. As a result, pharmaceutical companies are compelled to adopt robust cleaning validation protocols to meet these regulatory demands. This trend is expected to drive the market, as organizations invest in compliance solutions and validation services to adhere to these evolving standards.
Increased Focus on Quality Assurance
The heightened focus on quality assurance within the pharmaceutical sector is a significant driver of the Pharmaceutical Cleaning Validation Market. Companies are recognizing that effective cleaning validation is crucial for maintaining product quality and ensuring patient safety. This emphasis on quality assurance is leading to increased investments in cleaning validation processes and technologies. According to market analyses, the quality assurance segment is expected to witness substantial growth, as organizations prioritize compliance and risk management. This trend is likely to result in a greater demand for cleaning validation services, thereby propelling the Pharmaceutical Cleaning Validation Market forward.
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals is a pivotal driver in the Pharmaceutical Cleaning Validation Market. As biopharmaceuticals often require stringent cleaning validation processes to ensure product safety and efficacy, the market is witnessing a surge in the need for effective cleaning validation protocols. According to industry estimates, the biopharmaceutical sector is projected to grow at a compound annual growth rate of approximately 8% over the next few years. This growth necessitates enhanced cleaning validation measures to prevent cross-contamination and ensure compliance with regulatory standards. Consequently, companies are investing in advanced cleaning technologies and validation services, thereby propelling the Pharmaceutical Cleaning Validation Market forward.
Technological Innovations in Cleaning Processes
Technological innovations are transforming the Pharmaceutical Cleaning Validation Market, offering new solutions that enhance cleaning efficiency and validation accuracy. The advent of automated cleaning systems and advanced monitoring technologies allows for more precise validation processes, reducing the risk of human error. Moreover, the integration of data analytics and artificial intelligence in cleaning validation is gaining traction, enabling companies to optimize their cleaning protocols. These innovations not only improve compliance with regulatory standards but also enhance operational efficiency. As pharmaceutical companies increasingly adopt these technologies, the demand for cleaning validation services is likely to rise, further stimulating growth in the Pharmaceutical Cleaning Validation Market.
Growth of Contract Manufacturing Organizations (CMOs)
The expansion of Contract Manufacturing Organizations (CMOs) is influencing the Pharmaceutical Cleaning Validation Market. As pharmaceutical companies increasingly outsource their manufacturing processes to CMOs, the need for effective cleaning validation becomes paramount. CMOs must adhere to the same stringent cleaning validation standards as traditional manufacturers, driving demand for specialized cleaning validation services. The CMO market is projected to grow significantly, with estimates suggesting a compound annual growth rate of around 7% in the coming years. This growth is likely to create new opportunities for cleaning validation service providers, thereby enhancing the overall landscape of the Pharmaceutical Cleaning Validation Market.