Growing Focus on Patient Safety
The growing focus on patient safety is driving the Bioprocess Validation Market. As healthcare stakeholders prioritize patient outcomes, the need for validated bioprocesses becomes increasingly critical. Ensuring that biopharmaceutical products are produced consistently and safely is essential for maintaining public trust. This emphasis on patient safety is reflected in the increasing investments in validation processes by biopharmaceutical companies. According to industry reports, companies are allocating a significant portion of their budgets to validation activities to enhance product reliability. This trend indicates that the Bioprocess Validation Market is poised for growth as organizations recognize the importance of validation in safeguarding patient health and ensuring therapeutic efficacy.
Stringent Regulatory Requirements
Stringent regulatory requirements are a significant driver of the Bioprocess Validation Market. Regulatory agencies worldwide are imposing rigorous guidelines to ensure the safety and efficacy of biopharmaceutical products. For example, the International Conference on Harmonisation (ICH) has established guidelines that necessitate thorough validation of bioprocesses. This regulatory landscape compels biopharmaceutical companies to invest in validation processes to avoid potential penalties and product recalls. The increasing complexity of bioprocesses further necessitates comprehensive validation to meet these stringent standards. As a result, the Bioprocess Validation Market is likely to experience growth as companies seek to comply with evolving regulations and maintain their market position.
Emergence of Personalized Medicine
The emergence of personalized medicine is reshaping the Bioprocess Validation Market. As treatments become more tailored to individual patient needs, the complexity of bioprocesses increases. Personalized medicine often involves the use of advanced biopharmaceuticals, which require meticulous validation to ensure their safety and effectiveness. The rise of gene therapies and cell-based therapies exemplifies this trend, as these innovative treatments necessitate unique validation approaches. Consequently, the Bioprocess Validation Market is likely to expand as companies develop specialized validation protocols to accommodate the intricacies of personalized medicine. This shift not only enhances treatment outcomes but also underscores the critical role of validation in the evolving landscape of biopharmaceuticals.
Rising Demand for Biopharmaceuticals
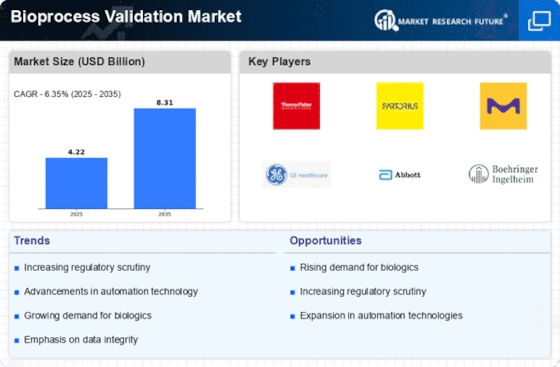
The increasing demand for biopharmaceuticals is a primary driver of the Bioprocess Validation Market. As the healthcare sector continues to evolve, there is a notable shift towards biologics, which are often more effective than traditional pharmaceuticals. This trend is evidenced by the fact that biopharmaceuticals accounted for approximately 30% of the total pharmaceutical market in recent years. Consequently, the need for robust bioprocess validation becomes paramount to ensure the safety and efficacy of these products. Regulatory bodies are emphasizing stringent validation processes to mitigate risks associated with biopharmaceutical production. This heightened focus on validation is likely to propel the growth of the Bioprocess Validation Market, as companies strive to meet compliance requirements and maintain product integrity.
Technological Advancements in Bioprocessing
Technological advancements in bioprocessing are significantly influencing the Bioprocess Validation Market. Innovations such as single-use technologies, automation, and advanced analytics are transforming traditional bioprocessing methods. For instance, the adoption of single-use systems has been reported to reduce contamination risks and enhance operational efficiency. Furthermore, the integration of automation in bioprocessing allows for real-time monitoring and control, which is essential for maintaining compliance with regulatory standards. As these technologies continue to evolve, they are expected to drive the demand for comprehensive validation processes. The Bioprocess Validation Market must adapt to these advancements to ensure that new technologies are effectively validated, thereby ensuring product quality and regulatory compliance.