North America : Market Leader in Innovation
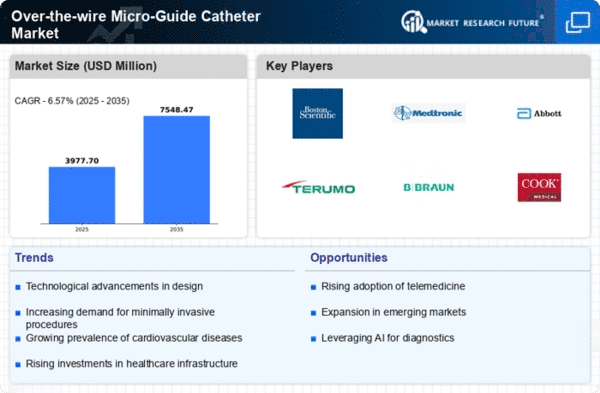
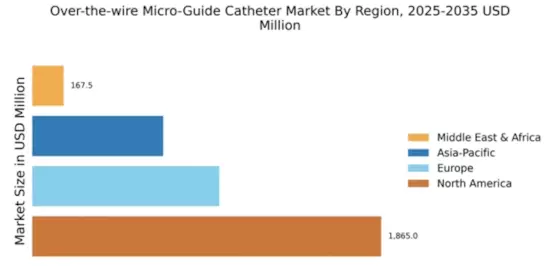
North America is poised to maintain its leadership in the Over-the-wire Micro-Guide Catheter market, holding a significant market share of $1865.0M in 2024. The region's growth is driven by advanced healthcare infrastructure, increasing prevalence of chronic diseases, and a strong focus on innovative medical technologies. Regulatory support from agencies like the FDA further catalyzes market expansion, ensuring safety and efficacy in medical devices.
The competitive landscape is robust, with key players such as Boston Scientific, Medtronic, and Abbott Laboratories leading the charge. The U.S. remains the largest market, supported by high healthcare spending and a growing aging population. The presence of established companies fosters innovation and enhances product offerings, ensuring that North America remains at the forefront of the micro-guide catheter market.
Europe : Emerging Market with Growth Potential
Europe's Over-the-wire Micro-Guide Catheter market is valued at $1000.0M, reflecting a growing demand for advanced medical devices. Factors such as an aging population, increasing healthcare expenditure, and a rise in minimally invasive procedures are driving market growth. Regulatory frameworks, including the EU Medical Device Regulation, ensure high standards for safety and efficacy, further boosting consumer confidence in these products.
Leading countries in this region include Germany, France, and the UK, where significant investments in healthcare technology are evident. Major players like B. Braun Melsungen AG and Terumo Corporation are expanding their presence, enhancing competition. The European market is characterized by a mix of established companies and innovative startups, fostering a dynamic environment for growth and development.
Asia-Pacific : Rapidly Growing Healthcare Sector
The Asia-Pacific region, with a market size of $700.0M, is rapidly emerging as a key player in the Over-the-wire Micro-Guide Catheter market. Factors such as increasing healthcare access, rising disposable incomes, and a growing prevalence of cardiovascular diseases are propelling market growth. Government initiatives aimed at improving healthcare infrastructure and regulatory reforms are also contributing to the positive market outlook.
Countries like Japan, China, and India are leading the charge, with significant investments in healthcare technology. Key players such as Asahi Intecc Co., Ltd. are expanding their operations to meet the rising demand. The competitive landscape is evolving, with both local and international companies vying for market share, making Asia-Pacific a vibrant hub for innovation in medical devices.
Middle East and Africa : Untapped Market with Opportunities
The Middle East and Africa (MEA) region, with a market size of $167.48M, presents significant growth potential in the Over-the-wire Micro-Guide Catheter market. Factors such as increasing healthcare investments, a rising prevalence of chronic diseases, and a growing focus on improving healthcare infrastructure are driving market dynamics. Regulatory bodies are also working towards enhancing the quality and safety of medical devices, which is crucial for market growth.
Countries like South Africa and the UAE are at the forefront of this growth, with increasing healthcare spending and a demand for advanced medical technologies. The presence of key players, although limited compared to other regions, is gradually increasing, creating a competitive landscape that is ripe for innovation and expansion.