Market Trends
Key Emerging Trends in the Over-the-wire Micro-Guide Catheter Market
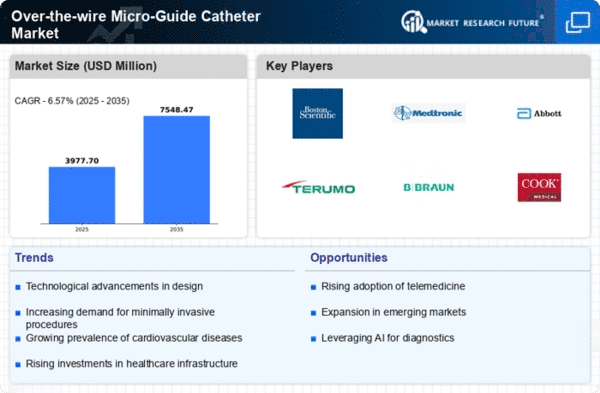
Growing Demand : The over-the-wire micro-guide catheter market is witnessing significant growth due to increasing demand for minimally invasive procedures in various medical specialties such as cardiology, neurology, and radiology.
Technological Advancements : Rapid advancements in technology, such as the development of innovative catheter designs and materials, are driving market growth. These advancements aim to enhance catheter performance, maneuverability, and patient outcomes.
Increasing Prevalence of Cardiovascular Diseases : The rising prevalence of cardiovascular diseases, coupled with the growing geriatric population worldwide, is fueling the demand for over-the-wire micro-guide catheters used in diagnostic and therapeutic procedures.
Expansion of Interventional Procedures : There is a growing trend towards the adoption of interventional procedures over traditional surgical techniques, leading to an increased utilization of micro-guide catheters for precise delivery of medical devices and therapies to target sites within the body.
Focus on Product Innovation : Key market players are focusing on continuous product innovation and development to introduce advanced micro-guide catheters with improved navigation capabilities, better trackability, and enhanced steerability, thereby catering to the evolving needs of healthcare providers.
Strategic Collaborations and Partnerships : Companies operating in the over-the-wire micro-guide catheter market are increasingly engaging in strategic collaborations and partnerships with other industry players, research institutions, and healthcare organizations to expand their product portfolio and geographical presence.
Rising Healthcare Expenditure : Increasing healthcare expenditure, especially in emerging economies, is contributing to the growth of the over-the-wire micro-guide catheter market by facilitating greater accessibility to advanced medical devices and technologies.
Regulatory Reforms and Quality Standards : Stringent regulatory reforms and adherence to quality standards imposed by regulatory authorities are influencing market dynamics by ensuring the safety, efficacy, and quality of micro-guide catheters, thereby fostering consumer trust and confidence.
Shift towards Outpatient Settings : There is a notable shift towards performing interventional procedures in outpatient settings rather than traditional hospital settings, driven by factors such as cost-effectiveness, convenience, and advancements in medical technology, which is expected to further propel market growth.
Geographical Expansion : Market players are focusing on expanding their geographical presence by penetrating untapped markets in regions with high unmet medical needs, such as Asia Pacific and Latin America, through distribution agreements, mergers, acquisitions, and strategic alliances.
















Leave a Comment