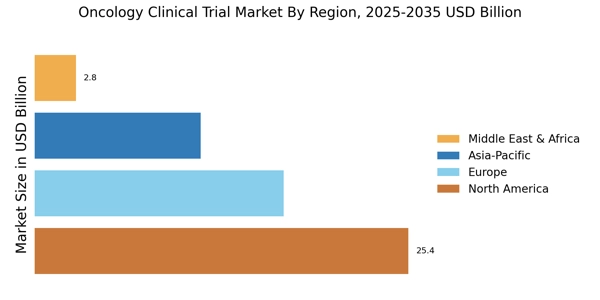
Advancements in Biotechnology
Advancements in biotechnology are transforming the landscape of the Oncology Clinical Trial Market. Innovations such as gene therapy, monoclonal antibodies, and CAR-T cell therapy are revolutionizing cancer treatment paradigms. The biotechnology sector has seen a surge in investment, with funding for cancer-related biotech startups reaching unprecedented levels. This influx of capital is facilitating the rapid development of novel therapies, which in turn drives the need for clinical trials to evaluate their efficacy and safety. As these biotechnological advancements continue to emerge, the Oncology Clinical Trial Market is poised for significant expansion, reflecting the growing intersection of technology and medicine.
Increasing Incidence of Cancer
The rising incidence of cancer worldwide is a primary driver of the Oncology Clinical Trial Market. According to recent statistics, cancer cases are projected to increase significantly, with estimates suggesting that by 2040, the number of new cancer cases could reach 29.5 million annually. This alarming trend necessitates the development of new therapies and treatment modalities, thereby propelling the demand for clinical trials. As pharmaceutical companies and research institutions strive to address this growing burden, the Oncology Clinical Trial Market is likely to experience substantial growth. The urgency to find effective treatments for various cancer types fosters an environment conducive to innovation and investment in clinical research.
Growing Investment in Cancer Research
The Oncology Clinical Trial Market is experiencing a surge in investment, driven by both public and private sectors. Governments and philanthropic organizations are increasingly allocating funds to cancer research initiatives, recognizing the critical need for innovative treatments. In recent years, funding for cancer research has reached billions of dollars, with substantial contributions from venture capitalists and pharmaceutical companies. This financial support is essential for conducting clinical trials, as it enables researchers to explore new therapeutic avenues and develop cutting-edge treatments. As investment continues to grow, the Oncology Clinical Trial Market is expected to thrive, fostering a robust pipeline of new therapies.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies, which serves as a catalyst for the Oncology Clinical Trial Market. Initiatives aimed at expediting the approval process for breakthrough therapies have been implemented, allowing for faster access to new treatments for patients. For instance, the FDA's Breakthrough Therapy Designation program has facilitated the development of numerous oncology drugs, significantly impacting the clinical trial landscape. This regulatory environment encourages pharmaceutical companies to invest in oncology research, thereby enhancing the Oncology Clinical Trial Market. The proactive stance of regulatory agencies is likely to foster a more dynamic and responsive clinical trial ecosystem.
Patient-Centric Approaches in Clinical Trials
The shift towards patient-centric approaches in clinical trials is reshaping the Oncology Clinical Trial Market. This paradigm emphasizes the importance of patient engagement and experience in the design and execution of clinical studies. By incorporating patient feedback and preferences, researchers can enhance recruitment and retention rates, ultimately leading to more successful trial outcomes. The adoption of digital tools and platforms facilitates better communication between patients and trial sponsors, further promoting participation. As the industry embraces these patient-centric methodologies, the Oncology Clinical Trial Market is likely to benefit from increased efficiency and improved trial results, aligning with the broader trend of prioritizing patient needs in healthcare.