Growing Emphasis on Patient Engagement
Patient engagement is emerging as a critical driver in the clinical trial-management-system market. As stakeholders recognize the importance of involving patients in the trial process, systems that facilitate communication and feedback are gaining traction. Enhanced patient engagement can lead to improved recruitment and retention rates, which are vital for trial success. In 2025, it is estimated that patient-centric approaches will account for over 30% of clinical trial strategies in the US. This shift indicates a growing recognition of the need for systems that prioritize patient experience, thereby influencing the design and functionality of clinical trial-management systems.
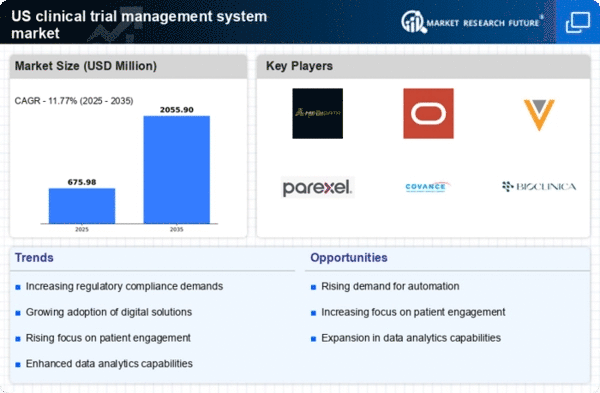
Rising Demand for Efficient Trial Management
The clinical trial-management-system market experiences a notable surge in demand for efficient trial management solutions. As the complexity of clinical trials increases, stakeholders seek systems that streamline processes, enhance collaboration, and improve data management. The market was projected to grow at a CAGR of approximately 12% from 2025 to 2030, driven by the need for faster drug development timelines and reduced operational costs. Organizations are increasingly adopting these systems to optimize resource allocation and ensure compliance with regulatory standards. This trend indicates a shift towards integrated solutions that can handle multiple aspects of trial management, thereby enhancing overall productivity in the clinical research landscape.
Regulatory Changes and Compliance Requirements
The clinical trial management system market is significantly impacted by evolving regulatory changes. Compliance requirements are also a major factor. As regulatory bodies implement stricter guidelines to ensure patient safety and data integrity, organizations must adapt their trial management processes accordingly. In 2025, compliance with these regulations is expected to drive the adoption of advanced clinical trial-management systems that can facilitate adherence to new standards. This trend highlights the necessity for systems that not only streamline trial operations but also ensure compliance with regulatory mandates, thereby influencing purchasing decisions in the market.
Focus on Data Analytics and Real-Time Monitoring
The clinical trial-management-system market is increasingly characterized by a focus on data analytics and real-time monitoring capabilities. As clinical trials generate vast amounts of data, the ability to analyze this information in real-time is becoming essential for decision-making. Organizations are investing in systems that provide advanced analytics tools to enhance trial efficiency and patient safety. The integration of data analytics into trial management systems is expected to improve patient recruitment and retention rates, ultimately leading to more successful trial outcomes. This trend suggests that the market will continue to evolve towards solutions that leverage data for strategic insights and operational improvements.
Increased Investment in Research and Development
Investment in research and development (R&D) within the pharmaceutical and biotechnology sectors significantly influences the clinical trial-management-system market. As companies allocate larger budgets to R&D, the need for robust trial management systems becomes paramount. In 2025, R&D spending in the US pharmaceutical industry is expected to exceed $100 billion, reflecting a commitment to innovation and the development of new therapies. This influx of funding necessitates the implementation of advanced clinical trial-management systems to manage the growing volume of data and ensure efficient trial execution. Consequently, the market is likely to benefit from this trend as organizations seek to maximize their R&D investments.