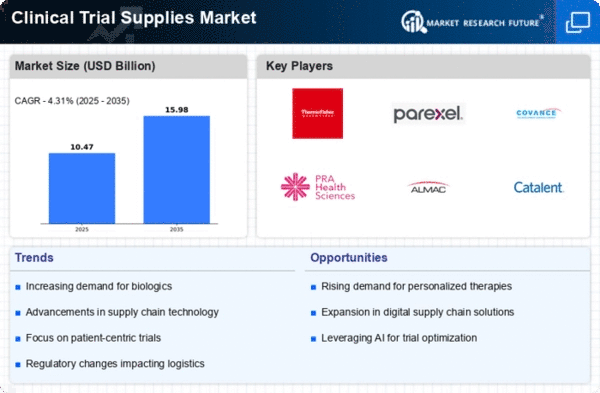
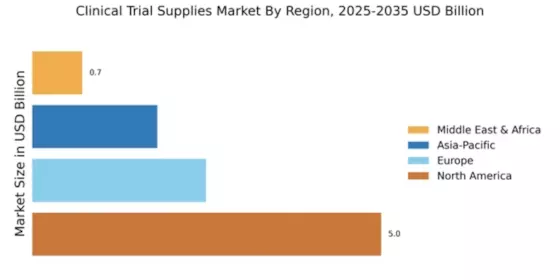
The Clinical Trial Supplies Market is currently experiencing a dynamic evolution, driven by the increasing complexity of clinical trials and the growing demand for innovative therapies. As pharmaceutical and biotechnology companies strive to expedite the development of new drugs, the need for efficient supply chain management and logistics solutions becomes paramount. This market is characterized by a diverse range of products, including investigational medicinal products, packaging materials, and ancillary supplies, all of which play a crucial role in ensuring the successful execution of clinical trials. Furthermore, the rise of personalized medicine and advanced therapies necessitates tailored supply solutions, which adds another layer of complexity to the market landscape.
In addition, the Clinical Trial Supplies Market is witnessing a shift towards digitalization and automation. Companies are increasingly adopting advanced technologies such as blockchain and artificial intelligence to enhance transparency and efficiency in supply chain operations. Various types of laboratories in the world provide essential analytical and diagnostic support for medical research. This trend not only streamlines processes but also mitigates risks associated with supply disruptions.
Moreover, regulatory compliance remains a critical factor influencing market dynamics, as organizations must navigate stringent guidelines to ensure the safety and efficacy of clinical trial materials. Overall, the Clinical Trial Supplies Market appears poised for continued growth, driven by innovation, technological advancements, and an unwavering commitment to improving patient outcomes.
Technological Advancements in Supply Chain Management
The integration of advanced technologies, such as artificial intelligence and blockchain, is transforming the Clinical Trial Supplies Market. Comprehensive clinical trial supply services include everything from initial planning to final site delivery. Consequently, organizations are better equipped to manage complex logistics and ensure compliance with regulatory standards. Innovation in clinical supply packaging is increasingly driven by the need for advanced temperature-controlled materials.
Patient-Centric Trial Designs
There is a noticeable shift towards patient-centric approaches in clinical trials, which necessitates the development of tailored supply strategies. This trend emphasizes the importance of accommodating diverse patient needs, potentially leading to more adaptive trial designs and customized supply solutions.
Regulatory Compliance and Quality Assurance
As regulatory frameworks become increasingly stringent, the Clinical Trial Supplies Market is likely to see a heightened focus on compliance and quality assurance. Companies may invest in specialized services to ensure that their supply chains meet the necessary standards, thereby safeguarding the integrity of clinical trials.
Regulatory Compliance
Global clinical trial manufacturing must strictly adhere to cGMP guidelines and regional laws to ensure the safety, quality, and efficacy. Robust regulatory compliance prevents costly delays and guarantees that investigational medicinal products are fit for their intended human use. The modernization of clinical trial supply systems is now focused on real-time visibility and end-to-end transparency due to strict regulations.
Strategic Logistics Integration
Effective clinical trial supply solutions streamline the distribution of investigational products. These systems enhance patient adherence by ensuring that specialized treatments reach global sites or homes with absolute diagnostic precision.