Collaboration Among Stakeholders
Collaboration among various stakeholders, including academic institutions, pharmaceutical companies, and research organizations, is becoming increasingly prevalent in the Omics-based Clinical Trial Market. These partnerships facilitate the sharing of knowledge, resources, and expertise, which is essential for advancing omics research. Collaborative initiatives often lead to the establishment of consortia that focus on specific therapeutic areas, thereby enhancing the efficiency of clinical trials. Such collaborations are likely to accelerate the pace of innovation and improve the overall success rates of omics-based studies. As the landscape of clinical research evolves, the importance of collaboration in the Omics-based Clinical Trial Market cannot be overstated.
Advancements in Omics Technologies
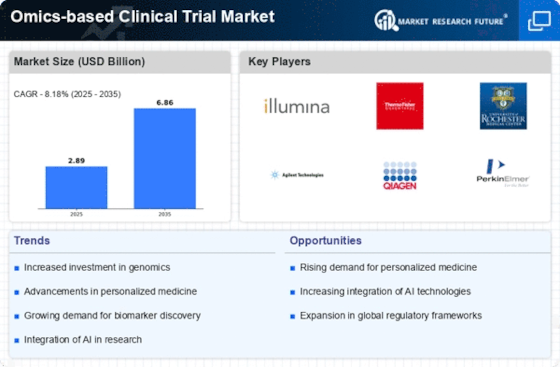
Technological advancements in omics disciplines, including genomics, transcriptomics, proteomics, and metabolomics, are significantly influencing the Omics-based Clinical Trial Market. Innovations such as next-generation sequencing (NGS) and high-throughput screening are enhancing the ability to analyze complex biological data. These advancements facilitate the identification of novel biomarkers and therapeutic targets, thereby improving the efficiency and success rates of clinical trials. The market for NGS alone is expected to grow at a compound annual growth rate (CAGR) of over 20% through 2026. As these technologies continue to evolve, they are likely to play a pivotal role in shaping the future of the Omics-based Clinical Trial Market.
Rising Investment in Biotechnology
The Omics-based Clinical Trial Market is witnessing a surge in investment from both public and private sectors, reflecting a growing interest in biotechnology. Venture capital funding for biotech firms has reached unprecedented levels, with investments exceeding USD 20 billion in recent years. This influx of capital is being directed towards research and development in omics technologies, which are seen as critical for advancing drug discovery and development processes. As biotechnology continues to attract significant financial resources, the Omics-based Clinical Trial Market is poised for substantial growth, driven by innovative research initiatives and the development of novel therapeutics.
Regulatory Support for Omics Research
Regulatory bodies are increasingly recognizing the potential of omics technologies in clinical research, which is fostering growth in the Omics-based Clinical Trial Market. Initiatives aimed at streamlining the approval processes for omics-based therapies and diagnostics are being implemented. For instance, the FDA has established guidelines for the use of genomic data in drug development, which encourages pharmaceutical companies to invest in omics research. This regulatory support not only enhances the credibility of omics-based studies but also accelerates the translation of research findings into clinical applications. As a result, the Omics-based Clinical Trial Market is likely to benefit from a more favorable regulatory landscape.
Increasing Demand for Precision Medicine
The Omics-based Clinical Trial Market is experiencing a notable surge in demand for precision medicine. This trend is largely driven by the growing recognition of the need for tailored therapeutic approaches that consider individual genetic profiles. As healthcare systems increasingly adopt personalized treatment strategies, the integration of omics technologies, such as genomics and proteomics, becomes essential. According to recent estimates, the precision medicine market is projected to reach USD 217 billion by 2028, indicating a robust growth trajectory. This demand for precision medicine is likely to propel the Omics-based Clinical Trial Market, as clinical trials increasingly focus on biomarker-driven patient selection and outcome prediction.