Focus on Preventive Healthcare
The growing emphasis on preventive healthcare is a crucial driver for the oem patient-monitoring-vital-sign-oem-module market. In Japan, healthcare policies are increasingly shifting towards preventive measures to reduce the burden of chronic diseases. This shift is reflected in the rising investments in health monitoring technologies that enable early detection and intervention. The Japanese government has allocated substantial funding to promote preventive healthcare initiatives, which is likely to boost the demand for advanced monitoring modules. As a result, manufacturers are expected to innovate and develop oem modules that support preventive care strategies, thereby expanding their market presence.
Growing Awareness of Health and Wellness
The rising awareness of health and wellness among the Japanese population is a notable driver for the oem patient-monitoring-vital-sign-oem-module market. As individuals become more health-conscious, there is a growing demand for devices that facilitate personal health monitoring. This trend is reflected in the increasing sales of wearable health devices and home monitoring systems. Market data suggests that the wearable health technology market in Japan is expected to grow at a CAGR of around 12% over the next few years. Consequently, this heightened awareness is likely to propel the development and adoption of innovative oem modules that cater to the needs of health-conscious consumers.
Rising Demand for Remote Monitoring Solutions
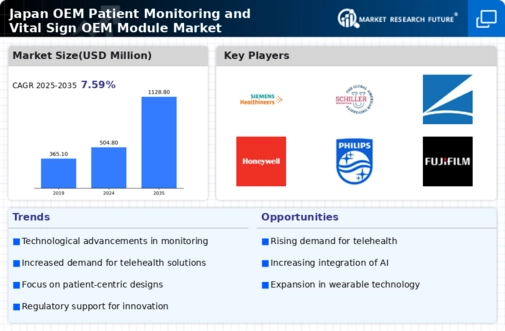
The increasing demand for remote monitoring solutions is a pivotal driver for the oem patient-monitoring-vital-sign-oem-module market. As healthcare providers in Japan seek to enhance patient care while minimizing hospital visits, the adoption of remote monitoring technologies is accelerating. This trend is particularly pronounced in chronic disease management, where continuous monitoring is essential. According to recent data, the market for remote patient monitoring devices is projected to grow at a CAGR of approximately 15% over the next five years. This growth is likely to spur the development and integration of advanced oem modules that facilitate seamless data transmission and real-time monitoring, thereby enhancing the overall efficiency of healthcare delivery in Japan.
Increased Investment in Healthcare Infrastructure
The ongoing investment in healthcare infrastructure in Japan is significantly influencing the oem patient-monitoring-vital-sign-oem-module market. The government has recognized the need to modernize healthcare facilities and improve service delivery, leading to increased funding for technological upgrades. Recent reports indicate that healthcare spending in Japan is projected to reach approximately $500 billion by 2026, with a substantial portion allocated to digital health solutions. This investment is likely to drive the demand for sophisticated oem modules that can integrate with existing systems, enhancing the overall quality of patient monitoring and care.
Integration of Artificial Intelligence in Healthcare
The integration of artificial intelligence (AI) into healthcare systems is emerging as a significant driver for the oem patient-monitoring-vital-sign-oem-module market. AI technologies are being utilized to analyze vast amounts of patient data, enabling healthcare providers to make informed decisions quickly. In Japan, the healthcare sector is increasingly adopting AI-driven solutions to improve diagnostic accuracy and patient outcomes. The market for AI in healthcare is expected to reach approximately $2 billion by 2026, indicating a robust growth trajectory. This trend suggests that oem modules equipped with AI capabilities will become essential components in patient monitoring systems, enhancing their functionality and effectiveness.



















Leave a Comment