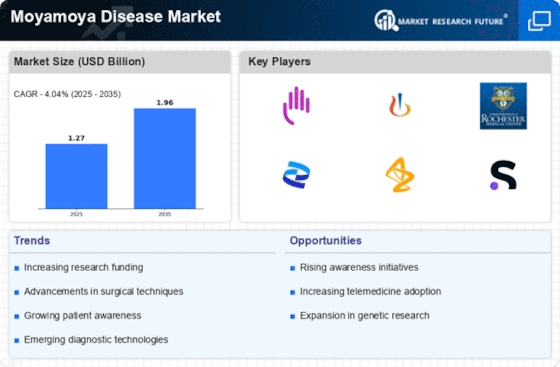
Enhanced Diagnostic Technologies
The Moyamoya Disease Market is benefiting from enhanced diagnostic technologies that facilitate earlier and more accurate detection of the disease. Advanced imaging techniques, such as magnetic resonance angiography (MRA) and computed tomography angiography (CTA), are becoming increasingly accessible and are crucial for timely diagnosis. The ability to identify Moyamoya disease at an earlier stage can lead to prompt intervention, which is essential for improving patient outcomes. As diagnostic capabilities improve, the number of diagnosed cases is likely to rise, thereby increasing the demand for treatment options within the Moyamoya Disease Market. This trend may also encourage healthcare providers to invest in state-of-the-art diagnostic equipment.
Advancements in Surgical Techniques
The Moyamoya Disease Market is witnessing significant advancements in surgical techniques, which are crucial for effective treatment. Procedures such as direct and indirect revascularization have evolved, leading to improved patient outcomes. These innovations not only enhance the safety and efficacy of surgeries but also reduce recovery times, making them more appealing to patients and healthcare providers alike. As surgical options become more refined, the demand for these procedures is likely to increase, thereby propelling the Moyamoya Disease Market forward. Furthermore, the integration of minimally invasive techniques may attract a broader patient base, contributing to market growth.
Increasing Incidence of Moyamoya Disease
The Moyamoya Disease Market is experiencing a notable increase in the incidence of this rare cerebrovascular disorder. Recent epidemiological studies indicate that the prevalence of Moyamoya disease is rising, particularly among certain demographics, such as individuals of Asian descent. This increase in cases is likely to drive demand for diagnostic and therapeutic options, thereby expanding the market. As healthcare providers become more aware of the disease, the number of diagnosed cases is expected to grow, leading to a greater need for specialized treatment facilities and resources. Consequently, this trend may stimulate investment in research and development, ultimately enhancing the Moyamoya Disease Market.
Growing Investment in Research and Development
Investment in research and development within the Moyamoya Disease Market is on the rise, driven by the need for better understanding and treatment of this complex condition. Pharmaceutical companies and research institutions are increasingly allocating resources to explore novel therapeutic agents and innovative treatment modalities. This influx of funding is expected to yield breakthroughs in drug development and clinical trials, potentially leading to more effective therapies for patients. As the scientific community continues to unravel the underlying mechanisms of Moyamoya disease, the market may see a surge in new products and treatment options, enhancing the overall landscape of the Moyamoya Disease Market.
Rising Awareness Among Healthcare Professionals
Rising awareness among healthcare professionals regarding Moyamoya disease is a pivotal driver for the Moyamoya Disease Market. As more clinicians become educated about the symptoms and implications of this rare condition, the likelihood of early diagnosis and appropriate treatment increases. Educational initiatives and training programs are being implemented to equip healthcare providers with the knowledge necessary to recognize and manage Moyamoya disease effectively. This heightened awareness is expected to lead to an increase in referrals to specialized centers, thereby boosting the demand for both diagnostic and therapeutic services. Consequently, this trend is likely to have a positive impact on the Moyamoya Disease Market.