Leading market players are undergoing various innovations activity to diversify their product & service offerings, which will help the Peripheral Artery Disease market, grow even more. Market participants are undertaking various strategic tactics to grow their presence, with key market developments including new product innovations, contracts & agreements, mergers & acquisitions, higher investments, partnerships, and collaboration with other organizations. To expand and survive in a more competitive and rising market climate, Peripheral Artery Disease industry must offer cost-effective items.
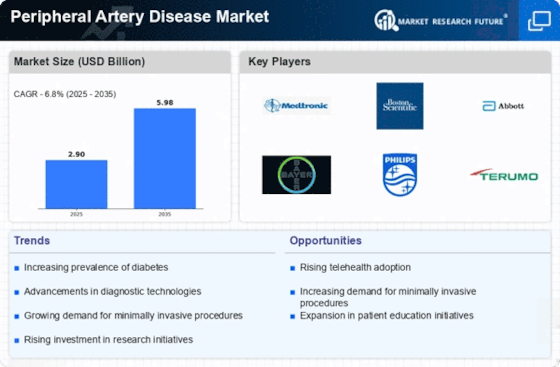
The producers are utilizing locally available raw materials to stand in the competition in the Peripheral Artery Disease industry to benefit clients and increase the market sector. In recent years, the Peripheral Artery Disease industry has offered some of the most significant advantages to medicine. Major players in the Peripheral Artery Disease market, including Philips N.V., Braun Melsungen AG, Medtronic, Bayer AG, Boston Scientific Corporation, Becton Dickinson and Company, Terumo Medical Corporation, Biotronik Cardinal Health., and others, are attempting to increase market demand by investing in research and development operations.
Becton Dickinson and Co. (BD) is a manufacturer and distributor of medical equipment, reagents, and instruments is. Syringes and pen needles, intravenous catheters, infusion pumps, disposables, automated medication dispensing and supply management systems, pre-fillable drug delivery systems, respiratory ventilation and diagnostic equipment and consumables, items for the secure collection and transportation of diagnostic specimens, instruments and reagent systems to detect a variety of infectious diseases and cancers, and clinical research tools are among its main products.
Researchers who are working in the branch of life sciences, hospitals, clinics, the pharmaceutical sector, and the general public can all benefit from BD's products.
In August The first-in-human trial of a peripheral sirolimus drug-coated balloon (DCB) has begun, according to BD (Becton, Dickinson and business) (NYSE BDX), a top medical technology business.
Koninklijke Philips NV (Philips) is a diversified technology firm that creates and produces consumer electronics and medical solutions. Diagnostic imaging, enterprise diagnostic informatics, image-guided therapy, ultrasound, monitoring and analytics, sleep and respiratory care, population health management, linked care informatics, and therapeutic care are all sectors in which the business provides products and solutions. Products available include power toothbrushes, brush heads, baby bottles, sterilisers, breast pumps, shavers, groomers, trimmers, and items for skin, hair, and hair removal. It also sells personal care and dental healthcare items.
In August 2020, A deal to acquire Intact Vascular, Inc., a company based in the United States that creates medical equipment for minimally invasive peripheral vascular operations, was announced by Royal Philips.