Expansion of Emerging Markets
Emerging markets are becoming increasingly significant players in the Medical Device Testing and Certification Market. As these regions experience economic growth and improvements in healthcare infrastructure, the demand for medical devices is on the rise. This growth presents opportunities for manufacturers to expand their reach, but it also necessitates compliance with local testing and certification requirements. The expansion into these markets may lead to increased competition, prompting companies to invest in robust testing processes to ensure product quality and safety. Additionally, the unique regulatory environments in emerging markets may drive the need for specialized testing services tailored to local standards. This trend suggests a dynamic shift in the market landscape, where emerging economies play a crucial role in shaping the future of medical device testing and certification.
Growing Focus on Patient Safety
Patient safety remains a paramount concern within the healthcare sector, significantly impacting the Medical Device Testing and Certification Market. As healthcare providers prioritize patient outcomes, the demand for high-quality medical devices has surged. This focus on safety compels manufacturers to adhere to rigorous testing and certification protocols to mitigate risks associated with device failures. The market is witnessing an increase in investments aimed at enhancing the reliability and performance of medical devices. Furthermore, the rise of patient advocacy groups has amplified the call for transparency in testing and certification processes, thereby influencing manufacturers to prioritize compliance. This trend indicates a potential shift towards more stringent testing standards, ultimately benefiting patient safety and device efficacy.
Stringent Regulatory Frameworks
The Medical Device Testing and Certification Market is significantly influenced by the evolving regulatory landscape. Regulatory bodies are continuously updating guidelines to ensure the safety and effectiveness of medical devices. This trend is particularly evident in regions where regulatory scrutiny has intensified, leading to more comprehensive testing requirements. Manufacturers are compelled to invest in testing and certification to meet these stringent standards, which may vary across different jurisdictions. The increasing complexity of regulations necessitates a thorough understanding of compliance processes, thereby driving demand for specialized testing services. As a result, the market for medical device testing and certification is likely to expand, as companies seek to navigate the intricate regulatory environment effectively.
Rising Demand for Medical Devices
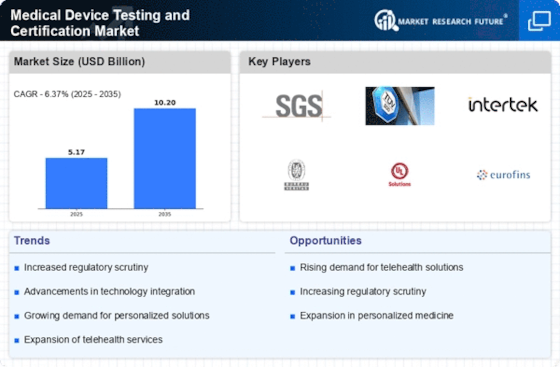
The increasing prevalence of chronic diseases and an aging population are driving the demand for medical devices. As healthcare systems evolve, there is a notable shift towards advanced medical technologies, which necessitates rigorous testing and certification. The Medical Device Testing and Certification Market is experiencing growth, with projections indicating a compound annual growth rate of approximately 6.5% over the next several years. This surge in demand compels manufacturers to ensure compliance with stringent regulatory standards, thereby enhancing the need for comprehensive testing and certification services. Furthermore, the expansion of telemedicine and home healthcare solutions is likely to contribute to the proliferation of medical devices, further emphasizing the importance of reliable testing and certification processes.
Technological Innovations in Testing Methods
Advancements in technology are revolutionizing the Medical Device Testing and Certification Market. Innovations such as artificial intelligence, machine learning, and automation are streamlining testing processes, enhancing accuracy, and reducing timeframes. These technologies enable more efficient data analysis and risk assessment, which are critical in ensuring the safety and efficacy of medical devices. As manufacturers increasingly adopt these cutting-edge technologies, the demand for specialized testing services is expected to rise. Moreover, the integration of digital tools in testing protocols may lead to improved compliance with regulatory requirements, thereby fostering a more robust certification process. This trend suggests a potential shift in the landscape of medical device testing, where technology plays a pivotal role in shaping industry standards.