Supportive Regulatory Framework
Japan's supportive regulatory framework for gene therapies plays a pivotal role in fostering market growth. The Pharmaceuticals and Medical Devices Agency (PMDA) has established guidelines that facilitate the approval process for innovative therapies. This regulatory environment encourages pharmaceutical companies to invest in gene therapy research and development, as it reduces the time and costs associated with bringing new treatments to market. In recent years, the PMDA has expedited the review process for several gene therapies, reflecting a commitment to enhancing patient access to cutting-edge treatments. As of November 2025, the gene therapy market is expected to benefit from these streamlined regulations, which may lead to an increase in the number of approved therapies. This supportive framework not only boosts investor confidence but also enhances collaboration between academia and industry, further driving innovation in the gene therapy market.
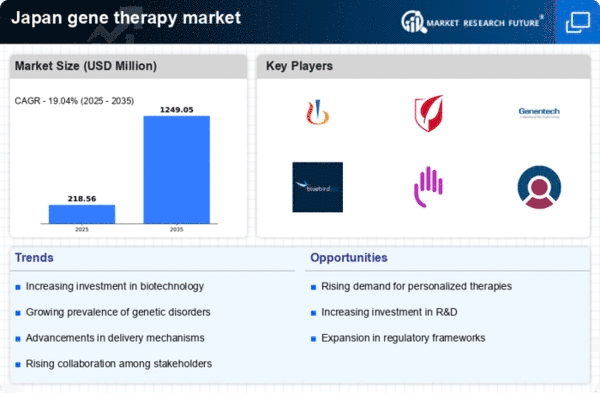
Increased Funding for Biotechnology
The gene therapy market in Japan is witnessing a notable increase in funding for biotechnology research. Government initiatives and private investments are channeling resources into the development of innovative therapies. In 2025, it is estimated that biotechnology funding in Japan has reached approximately ¥300 billion, with a significant portion allocated to gene therapy projects. This influx of capital is likely to accelerate research and development efforts, enabling companies to explore novel therapeutic approaches. Furthermore, partnerships between academic institutions and biotech firms are becoming more common, fostering an environment conducive to innovation. As funding continues to grow, the gene therapy market is expected to expand, with new therapies entering the pipeline and existing ones being refined. This trend indicates a robust future for the gene therapy market, driven by increased financial support and collaborative efforts.
Growing Prevalence of Genetic Disorders
The rising incidence of genetic disorders in Japan is a critical driver for the gene therapy market. Conditions such as muscular dystrophy, hemophilia, and certain types of cancer are becoming increasingly prevalent, necessitating innovative treatment solutions. Reports indicate that approximately 1 in 1,000 individuals in Japan is affected by a rare genetic disorder, highlighting the urgent need for effective therapies. The gene therapy market is positioned to address these challenges, offering potential cures rather than symptomatic treatments. As healthcare providers seek to improve patient outcomes, the demand for gene therapies is expected to rise significantly. This trend is further supported by the Japanese government's initiatives to promote research in rare diseases, which could lead to increased funding and resources for gene therapy development. Consequently, the growing prevalence of genetic disorders is likely to propel the gene therapy market forward.
Rising Demand for Personalized Medicine
The shift towards personalized medicine is significantly influencing the gene therapy market in Japan. Patients are increasingly seeking treatments tailored to their unique genetic profiles, which gene therapies are well-positioned to provide. This demand for individualized treatment options is prompting healthcare providers to explore gene therapies as viable solutions for various conditions. As of November 2025, the market is projected to grow as more patients recognize the potential benefits of personalized approaches. The integration of genomic testing into clinical practice is further facilitating this trend, allowing for more accurate diagnoses and targeted therapies. Additionally, the Japanese healthcare system is adapting to accommodate these advancements, which may lead to broader acceptance of gene therapies among practitioners and patients alike. Consequently, the rising demand for personalized medicine is likely to serve as a catalyst for growth in the gene therapy market.
Advancements in Genetic Engineering Techniques
The gene therapy market in Japan is experiencing a surge due to advancements in genetic engineering techniques. Innovations such as CRISPR and TALEN are revolutionizing the ability to edit genes with precision. This technological evolution enhances the efficacy of gene therapies, making them more appealing to healthcare providers and patients alike. As of 2025, the market is projected to grow at a CAGR of approximately 15%, driven by these advancements. The ability to target specific genetic disorders with tailored therapies is likely to increase the adoption of gene therapies in clinical settings. Furthermore, the integration of artificial intelligence in genetic research is expected to streamline the development process, thereby accelerating the introduction of new therapies into the market. This dynamic environment suggests a promising future for the gene therapy market in Japan.