Regulatory Support for Gene Therapies
Regulatory bodies are increasingly supportive of gene therapies, which is positively impacting the Cell and Gene Therapy Biomanufacturing Market. Initiatives aimed at expediting the approval process for innovative therapies are being implemented, allowing companies to bring their products to market more swiftly. For example, the introduction of breakthrough therapy designations and accelerated approval pathways has encouraged investment in gene therapy development. This regulatory environment not only fosters innovation but also enhances the confidence of investors and stakeholders in the biomanufacturing sector. As regulations continue to evolve, they are likely to create a more favorable landscape for the growth of the market.
Rising Prevalence of Genetic Disorders
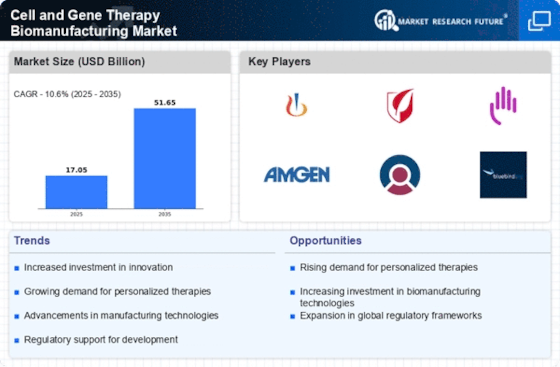
The increasing incidence of genetic disorders is a primary driver for the Cell and Gene Therapy Biomanufacturing Market. As more individuals are diagnosed with conditions such as hemophilia, cystic fibrosis, and muscular dystrophy, the demand for innovative therapies rises. According to recent estimates, genetic disorders affect approximately 1 in 10 individuals, leading to a significant need for effective treatment options. This growing patient population necessitates advancements in biomanufacturing processes to produce therapies at scale. Furthermore, the shift towards gene therapies that target the root causes of these disorders is likely to propel investments in biomanufacturing capabilities, thereby enhancing the overall market landscape.
Growing Demand for Personalized Medicine
The shift towards personalized medicine is a significant driver for the Cell and Gene Therapy Biomanufacturing Market. Patients increasingly seek treatments tailored to their unique genetic profiles, which necessitates the development of bespoke therapies. This trend is evident in the rising number of clinical trials focused on gene therapies that address specific genetic mutations. The market for personalized medicine is projected to grow substantially, with estimates suggesting a compound annual growth rate of over 10% in the coming years. As the demand for individualized treatments escalates, biomanufacturers are likely to adapt their processes to meet these needs, thereby enhancing their market position.
Advancements in Biomanufacturing Technologies
Technological innovations in biomanufacturing are transforming the Cell and Gene Therapy Biomanufacturing Market. The introduction of automated systems, improved cell culture techniques, and enhanced vector production methods are streamlining the manufacturing process. For instance, the adoption of single-use bioreactors has been shown to reduce production costs and time, making therapies more accessible. Additionally, advancements in CRISPR and other gene-editing technologies are facilitating the development of more precise and effective therapies. As these technologies continue to evolve, they are expected to drive efficiency and scalability in biomanufacturing, ultimately expanding the market's potential.
Increased Investment in Research and Development
Investment in research and development is a crucial driver for the Cell and Gene Therapy Biomanufacturing Market. Pharmaceutical companies and biotech firms are allocating substantial resources to explore novel therapies and improve existing ones. In recent years, R&D spending in the biopharmaceutical sector has surged, with estimates indicating a growth rate of over 5% annually. This influx of capital is fostering innovation in biomanufacturing processes, enabling the production of more complex and effective therapies. As companies strive to bring new products to market, the demand for advanced biomanufacturing solutions is likely to increase, further propelling market growth.