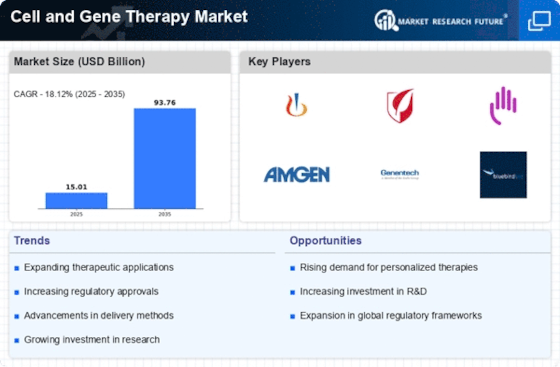
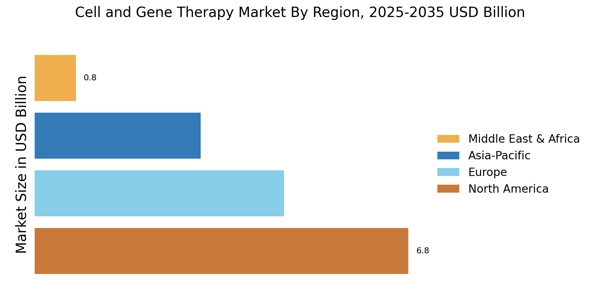
Rising Prevalence of Genetic Disorders
The increasing incidence of genetic disorders is a primary driver for the Cell and Gene Therapy Market. As more individuals are diagnosed with conditions such as hemophilia, cystic fibrosis, and muscular dystrophy, the demand for innovative therapies rises. According to recent estimates, approximately 1 in 1,500 individuals are affected by rare genetic disorders, highlighting a substantial patient population in need of effective treatments. This growing prevalence necessitates the development of targeted gene therapies, which aim to address the underlying genetic causes of these diseases. Consequently, pharmaceutical companies are investing heavily in research and development to create novel gene therapies, thereby propelling the Cell and Gene Therapy Market forward.
Growing Demand for Personalized Medicine
The shift towards personalized medicine is significantly influencing the Cell and Gene Therapy Market. Patients increasingly seek treatments tailored to their unique genetic profiles, which enhances therapeutic efficacy and minimizes adverse effects. This trend is supported by advancements in genomic sequencing technologies, which allow for comprehensive genetic analysis. As a result, the market for personalized therapies is expected to grow substantially, with projections indicating a value of USD 2 trillion by 2030. The emphasis on individualized treatment plans drives the development of gene therapies that can be customized to meet specific patient needs, thereby fostering innovation within the Cell and Gene Therapy Market.
Advancements in Gene Editing Technologies
Technological innovations in gene editing, particularly CRISPR and TALEN, are revolutionizing the Cell and Gene Therapy Market. These advancements enable precise modifications to DNA, facilitating the development of therapies that can effectively target and correct genetic mutations. The market for gene editing is projected to reach USD 10 billion by 2026, indicating a robust growth trajectory. As researchers continue to explore the potential of these technologies, the ability to create tailored therapies for various genetic disorders becomes increasingly feasible. This not only enhances the efficacy of treatments but also expands the scope of applications within the Cell and Gene Therapy Market, attracting significant investment and interest from stakeholders.
Increased Investment in Biopharmaceuticals
The surge in investment within the biopharmaceutical sector is a crucial driver for the Cell and Gene Therapy Market. Venture capital funding and public-private partnerships are on the rise, with investments exceeding USD 20 billion in recent years. This influx of capital is primarily directed towards the development of innovative therapies that address unmet medical needs. As companies strive to bring novel gene and cell therapies to market, the financial backing allows for extensive research, clinical trials, and regulatory compliance. This trend not only accelerates the pace of innovation but also enhances the overall growth potential of the Cell and Gene Therapy Market, as more therapies become available to patients.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of the development and approval of innovative therapies, which serves as a significant driver for the Cell and Gene Therapy Market. Initiatives aimed at expediting the approval process for breakthrough therapies have been implemented, allowing for faster access to life-saving treatments. For instance, the FDA's accelerated approval pathway has facilitated the introduction of several gene therapies in recent years. This regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that there is a streamlined process for bringing their products to market. As a result, the Cell and Gene Therapy Market is likely to experience accelerated growth, as more therapies gain regulatory approval and become available to patients.