Growth of Point-of-Care Testing
The growth of point-of-care testing (POCT) is emerging as a significant driver for the IVD Quality Control Market. POCT allows for rapid diagnostic results at the site of patient care, which is increasingly preferred in various healthcare settings. This trend is particularly relevant in emergency care and remote locations where timely decision-making is critical. As the demand for POCT rises, the need for stringent quality control measures becomes paramount to ensure the accuracy and reliability of these tests. The IVD Quality Control Market must adapt to support the unique challenges posed by POCT, including the need for portable quality control solutions. This shift is likely to stimulate innovation and investment in quality control technologies tailored for point-of-care applications, thereby expanding the market.
Increasing Regulatory Requirements
The IVD Quality Control Market is significantly influenced by the increasing regulatory requirements imposed by health authorities. Regulatory bodies are continuously updating guidelines to ensure the safety and efficacy of in vitro diagnostic products. Compliance with these regulations is essential for manufacturers to maintain market access and avoid penalties. The IVD Quality Control Market must adapt to these evolving standards, which often necessitate enhanced quality control measures. For example, the introduction of stricter quality assurance protocols can lead to increased operational costs for manufacturers. However, this also presents an opportunity for growth, as companies that invest in robust quality control systems are likely to gain a competitive edge. The market is expected to expand as organizations prioritize compliance and seek innovative solutions to meet regulatory demands.
Rising Demand for Accurate Diagnostics
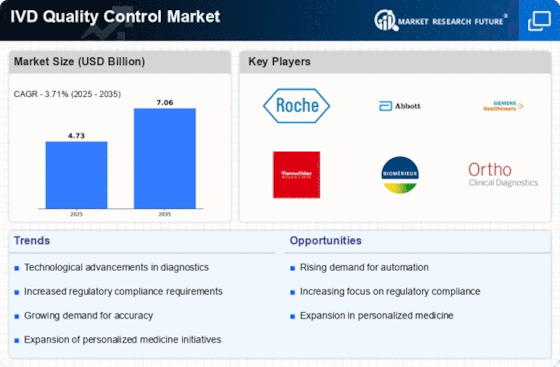
The increasing emphasis on accurate diagnostics is a primary driver for the IVD Quality Control Market. As healthcare systems strive for improved patient outcomes, the need for reliable and precise diagnostic tools has surged. This demand is reflected in the projected growth of the IVD market, which is expected to reach approximately 100 billion USD by 2025. The IVD Quality Control Market plays a crucial role in ensuring that diagnostic tests meet stringent accuracy standards, thereby enhancing the overall reliability of healthcare services. Furthermore, the rise in chronic diseases necessitates frequent testing, further propelling the need for robust quality control measures in IVD products. As a result, manufacturers are increasingly investing in quality control solutions to maintain compliance and meet the expectations of healthcare providers and patients alike.
Technological Innovations in Quality Control
Technological innovations are significantly influencing the IVD Quality Control Market. Advancements in automation, artificial intelligence, and data analytics are transforming quality control processes, making them more efficient and effective. For instance, automated quality control systems can reduce human error and enhance the speed of testing, which is vital in clinical settings. The integration of AI in quality control allows for real-time monitoring and predictive analytics, enabling proactive measures to be taken before issues arise. This shift towards technology-driven solutions is expected to contribute to the growth of the IVD Quality Control Market, as organizations seek to leverage these innovations to improve their operational efficiency and product reliability. As a result, the market is likely to witness an influx of new players offering cutting-edge quality control technologies.
Focus on Patient Safety and Quality Assurance
The focus on patient safety and quality assurance is a driving force behind the IVD Quality Control Market. As healthcare providers increasingly prioritize patient outcomes, the demand for high-quality diagnostic tests has intensified. This focus is reflected in the growing adoption of quality management systems that ensure the reliability of IVD products. The IVD Quality Control Market is responding to this trend by developing comprehensive quality assurance protocols that align with best practices in patient safety. Furthermore, the emphasis on quality assurance is likely to lead to increased collaboration between manufacturers and healthcare providers, fostering a culture of continuous improvement. As a result, the market is expected to grow as stakeholders recognize the importance of quality control in enhancing patient safety and overall healthcare quality.