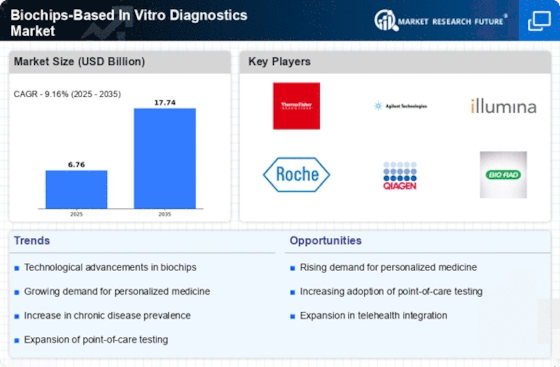
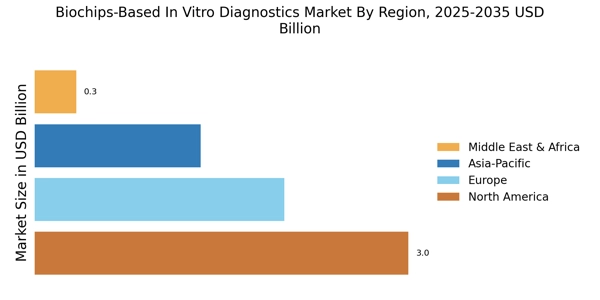
Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases such as diabetes, cancer, and cardiovascular disorders is a primary driver for the Biochips-Based In Vitro Diagnostics Market. As healthcare systems strive to manage these conditions effectively, the demand for rapid and accurate diagnostic tools has surged. Biochips offer the potential for high-throughput screening and real-time monitoring, which are essential for chronic disease management. According to recent estimates, the prevalence of diabetes alone is projected to reach 700 million by 2045, necessitating advanced diagnostic solutions. This trend indicates a growing market for biochips, as they can facilitate early detection and personalized treatment plans, thereby improving patient outcomes and reducing healthcare costs.
Advancements in Microfluidics Technology
Innovations in microfluidics technology are significantly enhancing the capabilities of the Biochips-Based In Vitro Diagnostics Market. Microfluidics allows for the manipulation of small volumes of fluids, enabling the development of more efficient and precise diagnostic devices. These advancements lead to improved sensitivity and specificity in detecting biomarkers, which is crucial for accurate diagnostics. The integration of microfluidics with biochips can potentially reduce the time and cost associated with traditional diagnostic methods. As a result, the market is witnessing a shift towards more compact, user-friendly devices that can be utilized in various settings, including point-of-care testing. This evolution is likely to attract investments and drive growth in the biochips sector.
Growing Demand for Point-of-Care Testing
The increasing preference for point-of-care testing (POCT) is a significant factor propelling the Biochips-Based In Vitro Diagnostics Market. POCT enables rapid diagnosis and immediate clinical decision-making, which is particularly valuable in emergency and remote settings. Biochips facilitate this trend by providing portable and efficient diagnostic solutions that can deliver results within minutes. The market for POCT is expected to expand, with estimates suggesting a compound annual growth rate of over 10% in the coming years. This growth is driven by the need for timely diagnosis in various medical fields, including infectious diseases and chronic conditions, thereby enhancing patient care and optimizing resource utilization.
Increased Investment in Research and Development
The Biochips-Based In Vitro Diagnostics Market is experiencing a surge in investment directed towards research and development (R&D). This influx of funding is primarily aimed at enhancing the capabilities of biochips and expanding their applications in diagnostics. As healthcare providers and research institutions recognize the potential of biochips in delivering precise and rapid results, they are increasingly investing in innovative technologies. This trend is reflected in the growing number of collaborations between academic institutions and biotech companies, which aim to develop next-generation biochips. The focus on R&D is likely to lead to breakthroughs that could redefine diagnostic standards and expand the market further.
Regulatory Support and Standardization Initiatives
Regulatory support and standardization initiatives are playing a crucial role in shaping the Biochips-Based In Vitro Diagnostics Market. Governments and regulatory bodies are increasingly recognizing the importance of biochips in enhancing diagnostic accuracy and patient care. Initiatives aimed at establishing clear guidelines and standards for biochip technologies are fostering innovation and ensuring safety and efficacy. This regulatory framework not only encourages manufacturers to invest in biochip development but also instills confidence among healthcare providers and patients. As a result, the market is likely to witness accelerated growth, driven by the assurance of quality and reliability in biochip-based diagnostics.