Regulatory Support for Innovation
Regulatory bodies are increasingly supportive of innovations within the Implantable Cardioverter Defibrillator Market (ICD) Market, facilitating the introduction of new technologies. Streamlined approval processes and incentives for research and development are encouraging manufacturers to invest in advanced ICD solutions. For instance, the FDA has established programs to expedite the review of breakthrough devices, which can significantly shorten the time to market for novel ICDs. This regulatory environment not only fosters innovation but also enhances competition among manufacturers, leading to improved product offerings. As a result, the ICD market is likely to witness a proliferation of new devices that cater to diverse patient needs, ultimately benefiting healthcare systems.
Increasing Awareness and Education
There is a growing awareness regarding the importance of early detection and treatment of cardiac conditions, which is positively influencing the Implantable Cardioverter Defibrillator Market (ICD) Market. Educational initiatives aimed at both healthcare professionals and patients are crucial in promoting the understanding of ICDs and their benefits. As more individuals become informed about the risks associated with arrhythmias and sudden cardiac arrest, the demand for ICD implantation is expected to rise. Additionally, healthcare providers are increasingly advocating for the use of ICDs as a preventive measure, further driving market growth. This heightened awareness is likely to translate into increased sales and adoption rates of ICDs in various healthcare settings.
Technological Advancements in ICDs
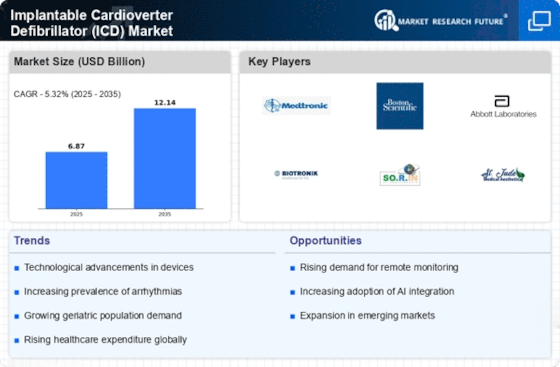
The Implantable Cardioverter Defibrillator Market (ICD) Market is experiencing a surge in technological advancements that enhance device functionality and patient outcomes. Innovations such as leadless ICDs and subcutaneous devices are gaining traction, offering less invasive options for patients. These advancements not only improve the safety profile of ICDs but also reduce the risk of complications associated with traditional leads. Furthermore, the integration of remote monitoring capabilities allows for real-time data transmission, enabling healthcare providers to make timely interventions. According to recent data, the market for advanced ICDs is projected to grow at a compound annual growth rate of over 8% through the next few years, reflecting the increasing demand for sophisticated cardiac care solutions.
Rising Cardiovascular Disease Incidence
The prevalence of cardiovascular diseases continues to rise, significantly impacting the Implantable Cardioverter Defibrillator Market (ICD) Market. Factors such as aging populations, sedentary lifestyles, and increasing rates of obesity contribute to this trend. As cardiovascular diseases become more common, the demand for effective treatment options, including ICDs, is expected to escalate. Recent statistics indicate that nearly 17.9 million people die from cardiovascular diseases each year, underscoring the urgent need for preventive and therapeutic measures. This growing patient population is likely to drive the ICD market, as healthcare providers seek to implement life-saving technologies to manage arrhythmias and prevent sudden cardiac arrest.
Aging Population and Healthcare Expenditure
The aging population is a significant driver of the Implantable Cardioverter Defibrillator Market (ICD) Market, as older adults are at a higher risk for cardiovascular diseases. As life expectancy increases, the number of individuals requiring cardiac interventions, including ICDs, is also expected to rise. Moreover, healthcare expenditure on chronic diseases is projected to increase, with a substantial portion allocated to cardiac care. This trend suggests that healthcare systems will prioritize investments in advanced technologies like ICDs to improve patient outcomes. Consequently, the ICD market is likely to expand, driven by the need to address the growing burden of cardiovascular diseases among the elderly.