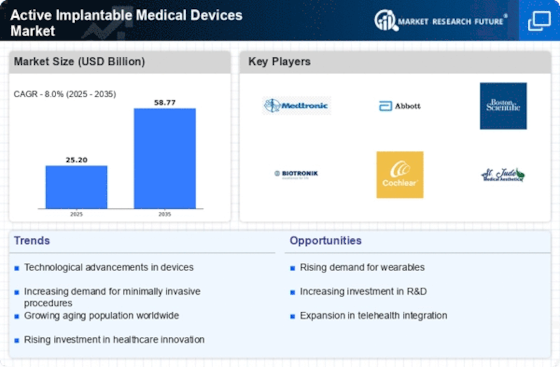
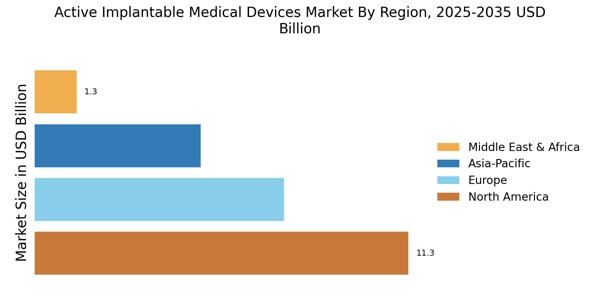
Increasing Healthcare Expenditure
The rise in healthcare expenditure across various regions is a crucial driver for the Active Implantable Medical Devices Market. As governments and private sectors allocate more funds towards healthcare, there is a corresponding increase in the adoption of advanced medical technologies, including active implantable devices. Reports indicate that healthcare spending is expected to reach trillions of dollars in the next few years, with a significant portion directed towards innovative medical solutions. This financial commitment facilitates the development and distribution of new implantable devices, thereby enhancing patient care and driving market growth. The correlation between healthcare investment and the demand for advanced medical devices is likely to remain strong.
Rising Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and neurological conditions is a primary driver of the Active Implantable Medical Devices Market. As these conditions become more common, the demand for advanced medical solutions rises. For instance, the World Health Organization indicates that cardiovascular diseases are the leading cause of death worldwide, necessitating the use of implantable devices like pacemakers and defibrillators. This trend suggests a growing market for active implantable devices, as healthcare providers seek innovative solutions to manage these chronic conditions effectively. Furthermore, the integration of these devices into patient care plans is likely to enhance treatment outcomes, thereby propelling market growth.
Technological Advancements in Device Design
Technological advancements in the design and functionality of active implantable medical devices are significantly influencing the Active Implantable Medical Devices Market. Innovations such as miniaturization, wireless connectivity, and enhanced biocompatibility are making these devices more effective and user-friendly. For example, the development of smart implantable devices that can monitor patient health in real-time is gaining traction. According to industry reports, the market for smart implantable devices is projected to grow at a compound annual growth rate of over 10% in the coming years. This technological evolution not only improves patient outcomes but also attracts investment and research, further stimulating market expansion.
Regulatory Support and Streamlined Approval Processes
Regulatory support and streamlined approval processes for active implantable medical devices are essential drivers of the Active Implantable Medical Devices Market. Regulatory bodies are increasingly adopting frameworks that facilitate faster approval of innovative devices, thereby encouraging manufacturers to invest in research and development. For instance, initiatives aimed at expediting the review process for breakthrough devices are becoming more common. This regulatory environment not only fosters innovation but also enhances market entry for new products. As a result, the availability of a wider range of implantable devices is likely to meet the diverse needs of patients, further propelling market growth.
Growing Awareness and Acceptance of Implantable Devices
The growing awareness and acceptance of implantable medical devices among patients and healthcare professionals are pivotal for the Active Implantable Medical Devices Market. Educational initiatives and successful case studies are helping to demystify these technologies, leading to increased patient willingness to consider implantable solutions. Surveys indicate that a significant percentage of patients are now more open to discussing implantable devices with their healthcare providers. This shift in perception is likely to drive demand, as more patients seek effective treatments for their conditions. Additionally, healthcare professionals are increasingly recognizing the benefits of these devices, which may lead to more frequent recommendations and prescriptions.