Market Growth Projections
Emergence of Direct-Acting Antivirals
The emergence of direct-acting antivirals (DAAs) has revolutionized the treatment landscape for Hepatitis C, significantly impacting the Global Hepatitis C Diagnosis and Treatment Market Industry. DAAs offer high cure rates and shorter treatment durations compared to traditional therapies, making them a preferred choice for patients and healthcare providers. The introduction of these innovative therapies has not only improved patient outcomes but also increased the overall treatment uptake. As a result, the market is witnessing a shift towards more effective treatment modalities, which is expected to drive growth in the coming years. The ongoing development of new DAAs further indicates a promising future for Hepatitis C treatment.
Advancements in Diagnostic Technologies
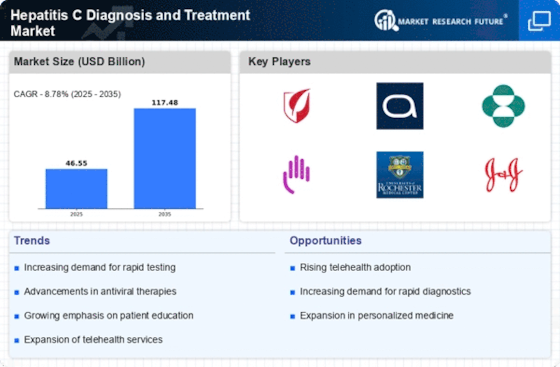
Technological advancements in diagnostic methods are transforming the landscape of the Global Hepatitis C Diagnosis and Treatment Market Industry. Innovations such as point-of-care testing and non-invasive diagnostic tools enhance the speed and accuracy of Hepatitis C detection. For instance, the development of rapid antibody tests and advanced molecular techniques allows for timely diagnosis, which is crucial for effective treatment initiation. These advancements not only improve patient outcomes but also facilitate broader screening initiatives, potentially reducing the burden of the disease. As the market evolves, the integration of these technologies is expected to contribute significantly to the anticipated growth, with projections indicating a market size of 117.4 USD Billion by 2035.
Rising Global Prevalence of Hepatitis C
The increasing prevalence of Hepatitis C globally serves as a primary driver for the Global Hepatitis C Diagnosis and Treatment Market Industry. As of 2024, approximately 71 million individuals are estimated to be living with chronic Hepatitis C worldwide. This growing patient population necessitates enhanced diagnostic and treatment solutions, thereby propelling market growth. The World Health Organization emphasizes the importance of early detection and effective treatment, which further underscores the need for innovative diagnostic technologies and antiviral therapies. Consequently, the market is projected to expand significantly, with a valuation of 46.5 USD Billion in 2024, reflecting the urgent demand for comprehensive Hepatitis C management.
Growing Awareness and Education Programs
The rising awareness regarding Hepatitis C and its implications is a crucial driver for the Global Hepatitis C Diagnosis and Treatment Market Industry. Educational programs aimed at informing the public about the risks, transmission, and treatment options for Hepatitis C are gaining momentum. Organizations and health authorities are actively promoting awareness campaigns that encourage individuals to seek testing and treatment. This heightened awareness is likely to lead to an increase in diagnosed cases, thereby driving demand for effective treatment options. As more individuals become informed about their health, the market is expected to experience substantial growth, aligning with the overall trend of increasing healthcare engagement.
Increased Government Initiatives and Funding
Government initiatives aimed at combating Hepatitis C are pivotal in driving the Global Hepatitis C Diagnosis and Treatment Market Industry. Many countries are implementing national strategies to eliminate Hepatitis C, which includes increased funding for screening programs and access to treatment. For example, the U.S. Centers for Disease Control and Prevention has launched campaigns to raise awareness and promote testing among high-risk populations. Such initiatives not only enhance public health outcomes but also stimulate market growth by increasing the demand for diagnostic and therapeutic solutions. The commitment to eradicate Hepatitis C is likely to sustain the market's upward trajectory, with a projected CAGR of 8.78% from 2025 to 2035.