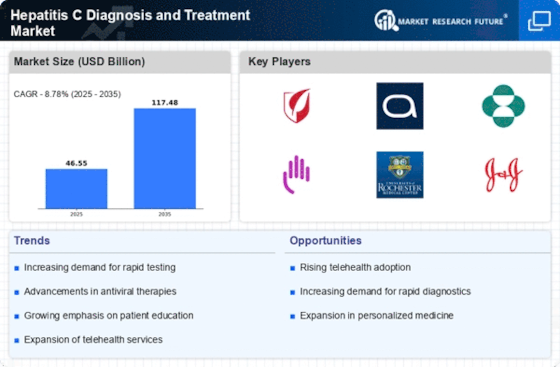
Top Industry Leaders in the Hepatitis C Diagnosis Treatment Market

Roche Launches New Gen-3 Hepatitis C Assay: November 2023, Roche unveiled Elecsys® Cobas® HCV Gen-3 Test, promising improved sensitivity and specificity for early detection of Hepatitis C infection.
Abbott Introduces Point-of-Care HCV Test: September 2023, Abbott's Alere™ i-Stat™ HCV Duo offered rapid on-site testing for both Hepatitis C antibodies and RNA, potentially facilitating faster diagnosis in low-resource settings.
Qiagen Expands Hepatitis C Genotyping Panel: October 2023, Qiagen's QIAamp® Viral RNA Mini Kit Plus now detects more HCV genotypes, aiding in selecting optimal treatment regimens.
Gilead Sciences' Sunlenso (lenacasvir/ribaravirin) Approved in Europe: November 2023 addition offers a short-course (8-week) treatment option for specific patient groups, increasing treatment accessibility and potentially boosting cure rates.
Merck & Co.'s Zepatier (grazoprevir/ritonavir) Expanded Label: December 2023, US approval for Zepatier broadened its use to previously untreated genotype 2 and 3 patients, offering another therapy option with high cure rates.
AbbVie Initiates Phase 3 Trial for Upadacitinib in Hepatitis C: December 2023, AbbVie's JAK inhibitor entered late-stage trials, potentially paving the way for a new class of medications for targeting viral replication.
List of Hepatitis C Diagnosis and Treatment Key Companies in the Market
- Hoffmann-La Roche Ltd (Switzerland)
- Vertex Pharmaceuticals Incorporated (US)
- Gilead Sciences Inc. (US)
- AbbVie Inc. (US)
- Novartis Pharmaceuticals Corporation (Switzerland)
- Bristol-Myers Squibb Company (US)
- Abbott (US)
- Beckman Coulter, Inc. (US)
- Siemens Medical Solutions USA Inc. (Germany)
- MedMira Inc. (Canada)
- GlaxoSmithKline PLC (UK)
- DiaSorin SpA (Italy)
- Qiagen (Germany)
- bioMérieux SA (France)
- Hologic Inc. (US)
- Bio-Rad Laboratories, Inc. (US)