Expansion of Treatment Options
The expansion of treatment options for hepatitis C is a crucial driver for the hepatitis c-diagnosis-treatment market. The introduction of new antiviral therapies, particularly direct-acting antivirals (DAAs), has revolutionized treatment protocols, offering higher cure rates and shorter treatment durations. As of now, these therapies are becoming increasingly accessible, with many insurance plans covering the costs. This accessibility is likely to encourage more patients to seek treatment, thereby increasing the overall market size. Furthermore, ongoing research and development efforts are expected to yield even more effective treatment options in the future, further propelling the hepatitis c-diagnosis-treatment market.
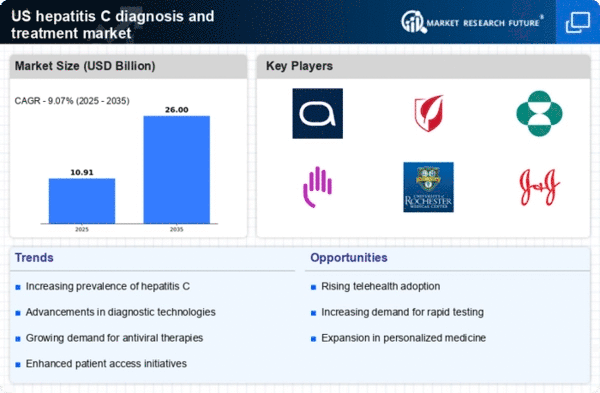
Rising Prevalence of Hepatitis C
The increasing prevalence of hepatitis C in the US is a critical driver for the hepatitis c-diagnosis-treatment market. As of recent estimates, approximately 2.4 million individuals are living with chronic hepatitis C in the US, highlighting a substantial patient population requiring diagnosis and treatment. This growing number necessitates enhanced screening and treatment options, thereby propelling market growth. The rising incidence is particularly notable among baby boomers, who are at a higher risk due to historical factors. Consequently, healthcare providers are focusing on improving diagnostic methods and treatment accessibility, which is likely to stimulate demand within the hepatitis c-diagnosis-treatment market.
Government Initiatives and Funding
Government initiatives aimed at combating hepatitis C are playing a pivotal role in shaping the hepatitis c-diagnosis-treatment market. Federal and state programs are increasingly allocating funds to enhance screening, treatment accessibility, and public awareness campaigns. For instance, the Centers for Disease Control and Prevention (CDC) has launched initiatives to promote hepatitis C testing among high-risk populations. Such efforts are likely to increase the number of diagnosed cases, subsequently boosting the demand for treatment options. The financial support from government entities is essential for developing new therapies and improving healthcare infrastructure, which could further stimulate market growth.
Innovations in Diagnostic Technologies
Technological advancements in diagnostic tools are significantly influencing the hepatitis c-diagnosis-treatment market. The introduction of highly sensitive and specific tests, such as polymerase chain reaction (PCR) and rapid diagnostic tests, has improved the accuracy of hepatitis C detection. These innovations facilitate early diagnosis, which is crucial for effective treatment outcomes. Moreover, the market is witnessing a shift towards point-of-care testing, allowing for immediate results and timely intervention. As healthcare systems increasingly adopt these advanced diagnostic technologies, the overall efficiency of hepatitis C management is expected to improve, thereby driving market growth.
Growing Demand for Preventive Healthcare
The rising emphasis on preventive healthcare is emerging as a significant driver for the hepatitis c-diagnosis-treatment market. As awareness of hepatitis C and its long-term health implications increases, more individuals are seeking preventive measures, including regular screenings. This trend is particularly evident among high-risk groups, such as individuals with a history of intravenous drug use or those who received blood transfusions before 1992. The proactive approach towards health management is likely to result in earlier diagnosis and treatment, thereby enhancing patient outcomes and driving market expansion. The hepatitis c-diagnosis-treatment market stands to benefit from this shift towards preventive healthcare.