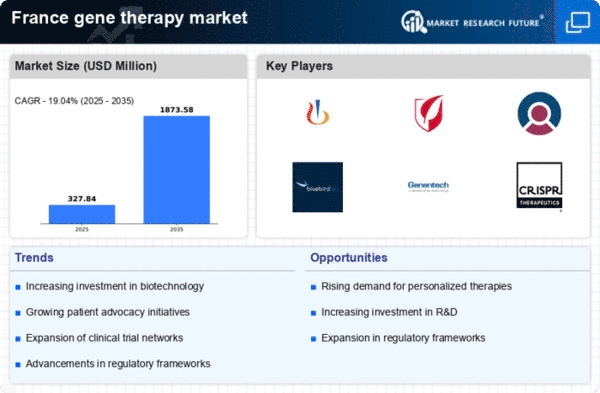
Increased Investment in Biotech
Investment in biotechnology is a key driver for the gene therapy market in France. In recent years, venture capital funding has significantly increased, with estimates suggesting that over €500 million was invested in biotech startups in 2024 alone. This influx of capital is likely to accelerate the development of innovative gene therapies. The gene therapy market is particularly attractive to investors due to its potential for high returns, especially as successful therapies gain regulatory approval. Additionally, public funding initiatives aimed at fostering biotech innovation further bolster this investment landscape. As a result, the market is poised for substantial growth, with new therapies expected to enter the market in the coming years.
Advancements in Genetic Research
The gene therapy market in France is experiencing a surge due to advancements in genetic research. Innovations in CRISPR technology and other gene-editing techniques are paving the way for more effective therapies. As of 2025, the market is projected to reach approximately €1.5 billion, reflecting a growth rate of around 15% annually. This growth is driven by the increasing understanding of genetic disorders and the potential for targeted treatments. The gene therapy market is benefiting from collaborations between academic institutions and biotech firms, which are crucial for translating research into viable therapies. Furthermore, the French government is investing in research initiatives, which enhances the overall landscape for gene therapy development.
Regulatory Framework Enhancements
The regulatory framework surrounding gene therapy in France is evolving, which is likely to facilitate market growth. Recent reforms have streamlined the approval process for gene therapies, reducing the time it takes for new treatments to reach patients. The gene therapy market is benefiting from these enhancements, as they encourage more companies to invest in research and development. As of November 2025, the average time for regulatory approval has decreased by approximately 20%, which is expected to lead to a higher number of therapies entering the market. This regulatory support not only fosters innovation but also instills confidence among investors and stakeholders in the gene therapy sector.
Public Health Initiatives and Funding
Public health initiatives in France are increasingly focusing on genetic research and therapy, which serves as a catalyst for the gene therapy market. The French government has allocated substantial funding towards healthcare innovation, with a reported €300 million dedicated to genetic research in 2025. These initiatives aim to enhance the healthcare system's capacity to address genetic disorders effectively. The gene therapy market is likely to benefit from this funding, as it supports the development of new therapies and the establishment of research centers. Furthermore, public awareness campaigns are educating the population about genetic disorders, which may lead to increased demand for gene therapies, thereby driving market growth.
Growing Prevalence of Genetic Disorders
The rising prevalence of genetic disorders in France is a significant driver for the gene therapy market. Conditions such as hemophilia, muscular dystrophy, and certain types of cancer are becoming increasingly common, necessitating the development of effective treatments. Current estimates indicate that approximately 1 in 2000 individuals are affected by rare genetic disorders, highlighting a substantial patient population in need of innovative therapies. The gene therapy market is responding to this demand by focusing on developing targeted therapies that address specific genetic mutations. This focus not only aims to improve patient outcomes but also aligns with the broader healthcare goal of personalized medicine, which is gaining traction in France.