Regulatory Compliance and Standards
The Cell And Gene Therapy Manufacturing Quality Control QC Market is significantly influenced by the stringent regulatory compliance and standards imposed by health authorities. Regulatory bodies are increasingly emphasizing the need for robust quality control systems to ensure the safety and efficacy of cell and gene therapies. As of October 2025, manufacturers are required to adhere to comprehensive guidelines that dictate every aspect of the production process, from raw material sourcing to final product testing. This regulatory landscape compels companies to invest in advanced QC systems and personnel training, thereby driving growth in the QC market. The ongoing evolution of these regulations suggests that manufacturers must remain agile and responsive to maintain compliance and avoid potential penalties.
Rising Demand for Advanced Therapies
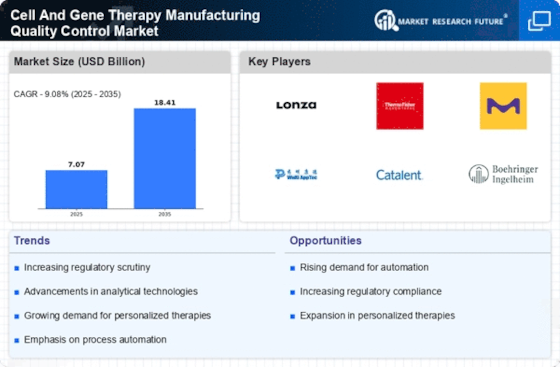
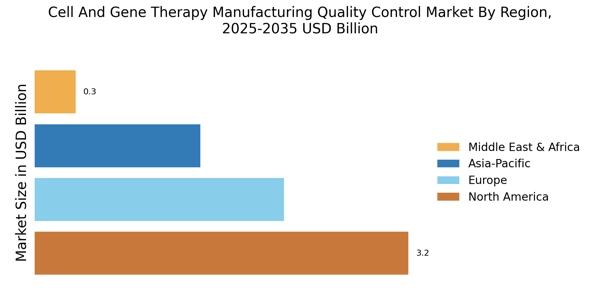
The Cell And Gene Therapy Manufacturing Quality Control QC Market is experiencing a notable surge in demand for advanced therapies. This increase is largely driven by the growing prevalence of genetic disorders and chronic diseases, which necessitate innovative treatment solutions. As of 2025, the market for cell and gene therapies is projected to reach substantial figures, reflecting a compound annual growth rate that underscores the urgency for effective quality control measures. The need for rigorous QC processes is paramount to ensure the safety and efficacy of these therapies, thereby fostering trust among healthcare providers and patients alike. Consequently, manufacturers are compelled to invest in sophisticated QC technologies and methodologies to meet regulatory standards and patient expectations.
Growing Focus on Patient-Centric Approaches
The Cell And Gene Therapy Manufacturing Quality Control QC Market is witnessing a growing focus on patient-centric approaches, which is reshaping quality control practices. As healthcare shifts towards personalized medicine, the need for therapies tailored to individual patient needs becomes paramount. This trend necessitates enhanced quality control measures to ensure that therapies are not only effective but also safe for diverse patient populations. By October 2025, it is expected that manufacturers will increasingly prioritize patient feedback and outcomes in their QC processes, leading to more adaptive and responsive quality control systems. This patient-centric focus may drive innovation in QC methodologies, ultimately enhancing the overall quality of cell and gene therapies.
Technological Advancements in Quality Control
Technological advancements are playing a pivotal role in shaping the Cell And Gene Therapy Manufacturing Quality Control QC Market. Innovations such as automation, artificial intelligence, and machine learning are enhancing the efficiency and accuracy of quality control processes. These technologies enable real-time monitoring and analysis of production processes, which is crucial for maintaining compliance with stringent regulatory requirements. As the industry evolves, the integration of these advanced technologies is expected to streamline QC operations, reduce human error, and ultimately lower production costs. The adoption of such technologies is likely to become a key differentiator among manufacturers, influencing their competitive positioning in the market.
Increased Investment in Research and Development
Investment in research and development is a critical driver for the Cell And Gene Therapy Manufacturing Quality Control QC Market. As companies strive to innovate and develop new therapies, the demand for effective quality control measures becomes increasingly pronounced. In 2025, it is anticipated that R&D expenditures in the field of cell and gene therapy will reach unprecedented levels, reflecting a commitment to advancing therapeutic options. This influx of investment not only supports the development of novel therapies but also necessitates the establishment of rigorous QC protocols to ensure that these therapies meet safety and efficacy standards. Consequently, the interplay between R&D and QC is likely to shape the future landscape of the industry.