-
Executive summary
-
Market Introduction
-
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
Research Methodology
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
- Primary Interviews and Information Gathering Process
- Breakdown of Primary Respondents
-
Forecasting Model
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
-
Data Triangulation
-
Validation
-
Market Dynamics
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
Market Factor Analysis
-
Value Chain Analysis
-
Porter’s Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Regional Impact
- Opportunity and Threat Analysis
-
GLOBAL C REACTIVE PROTEIN TESTING MARKET, BY Assay Type
-
Overview
-
Enzyme-linked immunosorbent assay (ELISA)
-
Chemiluminescence Immunoassay (CLIA)
-
Immunoturbidimetric assays
-
GLOBAL C REACTIVE PROTEIN TESTING MARKET, BY Application
-
Diabetes
-
Rheumatoid Arthritis
-
Cardiovascular Disease
-
Inflammatory Bowel Disease
-
Others
-
GLOBAL C REACTIVE PROTEIN TESTING MARKET, by Region
-
Overview
-
North America
- US
- Canada
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
-
Asia-Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia-Pacific
-
Rest of the World
- Middle East
- Africa
- Latin America
-
Competitive Landscape
-
Overview
-
Competitive Analysis
-
Market Share Analysis
-
Major Growth Strategy in the Global C reactive protein testing Market,
-
Competitive Benchmarking
-
Leading Players in Terms of Number of Developments in the Global C reactive protein testing Market,
-
Key developments and Growth Strategies
- New Product Launch/Service Deployment
- Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales & Operating Income, 2022
- Major Players R&D Expenditure. 2022
-
Company ProfileS
-
Randox Laboratories Limited
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Merck KGAA (Millipore Sigma)
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Quest Diagnostics Incorporated
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Abbott Laboratories
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Zoetis Inc. (Abaxis Inc.)
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Thermo Fisher Scientific
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Danaher Corporation (Beckman Coulter Inc.)
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
HORIBA, LTD
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
F. Hoffmann-La Roche AG
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Laboratory Corporation of America Holdings
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Appendix
-
References
-
Related Reports LIST OF TABLES
-
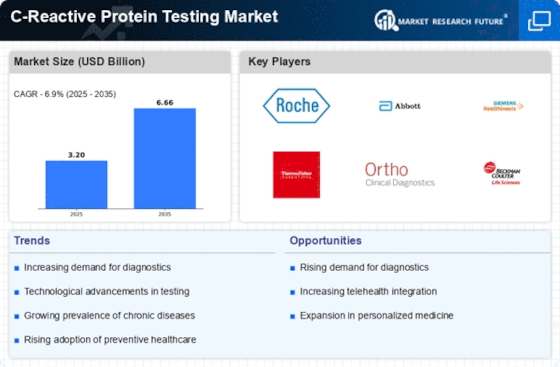
Global C reactive protein testing Market, Synopsis, 2018-2032
-
Global C reactive protein testing Market, Estimates & Forecast, 2018-2032 (USD BILLION)
-
GLOBAL C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
GLOBAL C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
North America: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
North America: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
US: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
US: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
Canada: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
Canada: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
Europe: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
Europe: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
germany: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
germany: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
FRANCE: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
FRANCE: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
italy: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
italy: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
spain: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
spain: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
UK: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
UK: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
rest of europe: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
rest of europe: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
Asia-Pacific: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
Asia-Pacific: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
japan: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
japan: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
japan: C REACTIVE PROTEIN TESTING MARKET, BY End User, 2018-2032 (USD BILLION)
-
china: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
china: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
india: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
TABLE 23
-
india: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
australia: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
australia: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
south korea: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
south korea: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
rest of asia-pacific: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
rest of asia-pacific: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
rest of the world: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
rest of the world: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
Middle east: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
Middle east: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
Africa: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
Africa: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION)
-
Latin america: C REACTIVE PROTEIN TESTING MARKET, BY Assay Type, 2018-2032 (USD BILLION)
-
Latin america: C REACTIVE PROTEIN TESTING MARKET, BY Application, 2018-2032 (USD BILLION) LIST OF FIGURES
-
Research Process
-
Market Structure for the Global C reactive protein testing Market
-
Market Dynamics for the Global C reactive protein testing Market
-
Global C reactive protein testing Market, Share (%), BY Assay Type, 2022
-
Global C reactive protein testing Market, Share (%), BY Application, 2022
-
Global C reactive protein testing Market, Share (%), by Region, 2022
-
north AMERICA: C REACTIVE PROTEIN TESTING MARKET, SHARE (%), BY REGION, 2022
-
Europe: C REACTIVE PROTEIN TESTING MARKET, SHARE (%), BY REGION, 2022
-
Asia-Pacific: C REACTIVE PROTEIN TESTING MARKET, SHARE (%), BY REGION, 2022
-
Rest of the world: C REACTIVE PROTEIN TESTING MARKET, SHARE (%), BY REGION, 2022
-
Global C reactive protein testing Market: Company Share Analysis, 2022 (%)
-
Randox Laboratories Limited: FINANCIAL OVERVIEW SNAPSHOT
-
Randox Laboratories Limited: SWOT ANALYSIS
-
MERCK KGAA (MILLIPORE SIGMA): FINANCIAL OVERVIEW SNAPSHOT
-
MERCK KGAA (MILLIPORE SIGMA): SWOT ANALYSIS
-
Quest Diagnostics Incorporated: FINANCIAL OVERVIEW SNAPSHOT
-
Quest Diagnostics Incorporated: SWOT ANALYSIS
-
Abbott Laboratories: FINANCIAL OVERVIEW SNAPSHOT
-
Abbott Laboratories: SWOT ANALYSIS
-
ZOETIS INC. (ABAXIS INC.).: FINANCIAL OVERVIEW SNAPSHOT
-
ZOETIS INC. (ABAXIS INC.).: SWOT ANALYSIS
-
Thermo Fisher Scientific: FINANCIAL OVERVIEW SNAPSHOT
-
Thermo Fisher Scientific: SWOT ANALYSIS
-
Danaher Corporation (Beckman Coulter Inc.): FINANCIAL OVERVIEW SNAPSHOT
-
Danaher Corporation (Beckman Coulter Inc.): SWOT ANALYSIS
-
HORIBA, LTD: FINANCIAL OVERVIEW SNAPSHOT
-
HORIBA, LTD: SWOT ANALYSIS
-
F. Hoffmann-La Roche AG: FINANCIAL OVERVIEW SNAPSHOT
-
F. Hoffmann-La Roche AG: SWOT ANALYSIS
-
Laboratory Corporation of America Holdings: FINANCIAL OVERVIEW SNAPSHOT
-
Laboratory Corporation of America Holdings: SWOT ANALYSIS

















Leave a Comment