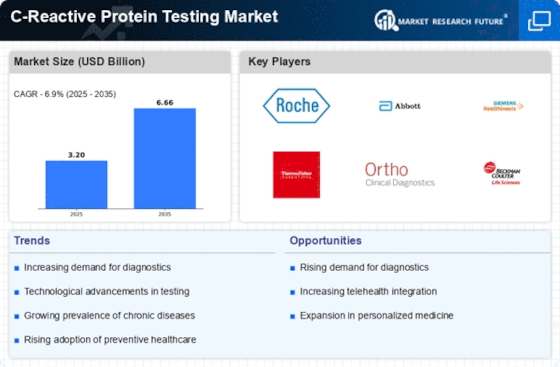
Top Industry Leaders in the C Reactive Protein Testing Market
 Latest C reactive protein testing Companies Update
Latest C reactive protein testing Companies Update
Jan 2024Siemens Healthineers introduced their Atellica® CH 900 System featuring a new high-sensitivity CRP assay for earlier detection of low-grade inflammation associated with chronic diseases.Collaborated with research institutions to explore the potential of CRP testing in personalized medicine approaches for tailoring treatment strategies based on individual inflammatory profiles.
Roche Diagnostics launched their Elecsys® CRP Ultrasensitive assay, offering improved sensitivity for early detection of subtle inflammatory changes.Partnered with healthcare providers to offer CRP testing as part of comprehensive wellness programs for early disease risk identification and prevention.
Abbott Laboratories received FDA approval for their Alinity™ L i-CRP assay, a rapid and sensitive point-of-care CRP test suitable for use in a clinical setting.Focused on expanding their POC CRP testing offerings to improve accessibility and timely diagnosis of inflammatory conditions.
Randox Laboratories developed their Biochip Array Technology (BAT) platform for simultaneous measurement of multiple inflammatory markers, including CRP, offering a more comprehensive assessment of systemic inflammation.Partnered with laboratories and clinical research centers to implement BAT-based testing for improved diagnosis and research of inflammatory diseases.
IQVIA (QuintilesIMS) published a report on the global CRP testing market, analyzing its growth potential, key players, and regional trends.Highlighted the increasing adoption of POC CRP testing in primary care settings as a significant market driver.List of C Reactive Protein Testing Key Companies in the Market
- Randox Laboratories Limited
- Merck KGAA (Millipore Sigma)
- Quest Diagnostics Incorporated
- Abbott Laboratories
- Zoetis Inc. (Abaxis Inc.)
- Thermo Fisher Scientific, Inc.
- Danaher Corporation (Beckman Coulter Inc.)
- Horiba, Ltd.
- Laboratory Corporation of America Holdings
- F. Hoffmann-La Roche AG