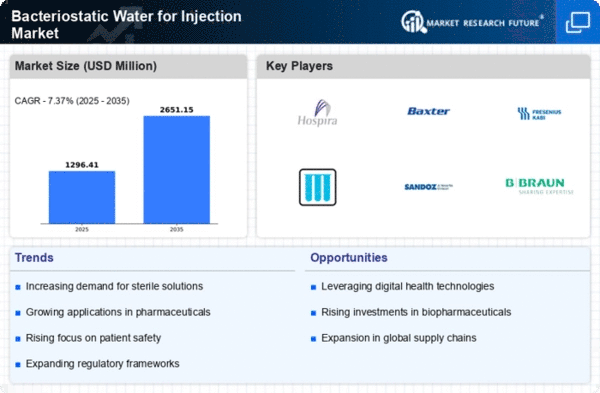
Top Industry Leaders in the Bacteriostatic Water for Injection Market

Dec 2023Acella Pharmaceuticals, LLC received a renewed cGMP certificate for their FDA-approved BWFI facility, demonstrating continued commitment to high-quality sterile product manufacturing.Partnered with a leading pharmaceutical company to supply BWFI for their injectable medication production.
Merck KGaA launched a new line of pre-filled syringes containing BWFI and various medications, simplifying drug administration and reducing medication preparation errors.Received market authorization for their BWFI product in several new countries, expanding their global reach.
Pfizer announced investments in their BWFI production facility to increase capacity and meet the growing demand for their injectable medications.Implemented sustainable practices in their BWFI production, reducing water and energy consumption.
Reckitt Benckiser Group Collaborated with academic institutions on research projects exploring the development of novel antibacterial preservatives for BWFI, potentially enhancing its shelf life and safety.Introduced a new online platform for ordering and tracking BWFI deliveries, improving customer service and supply chain efficiency.
List of Bacteriostatic Water for Injection Key Companies in the Market
- Acella Pharmaceuticals, LLC
- Merck KGaA
- Abbott Laboratories
- Procter and Gamble
- Pfizer, Inc.
- Reckitt Benckiser Group PLC
- Novartis AG
- GlaxoSmithKline PLC
- Sanofi
- Johnson and Johnson Services, Inc.