Supportive Regulatory Environment
A supportive regulatory environment is emerging as a crucial driver for the Autologous Stem Cell Therapies Market. Regulatory agencies are increasingly recognizing the potential of autologous therapies and are streamlining approval processes to facilitate their development and commercialization. Recent initiatives aimed at expediting the review of regenerative medicine products suggest a commitment to fostering innovation in this field. This regulatory support not only enhances the feasibility of bringing new therapies to market but also instills confidence among investors and stakeholders. As a result, the Autologous Stem Cell Therapies Market is likely to benefit from a more favorable regulatory landscape, encouraging further advancements and broader access to these therapies.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases such as diabetes, cardiovascular disorders, and neurodegenerative conditions appears to be a primary driver for the Autologous Stem Cell Therapies Market. As these diseases become more prevalent, the demand for innovative treatment options intensifies. According to recent data, chronic diseases account for approximately 70% of all deaths worldwide, highlighting the urgent need for effective therapies. Autologous stem cell therapies, which utilize the patient's own cells, offer a promising avenue for treatment, potentially reducing the risk of rejection and complications. This trend suggests that as the population ages and the burden of chronic diseases increases, the Autologous Stem Cell Therapies Market is likely to experience substantial growth.
Growing Investment in Regenerative Medicine
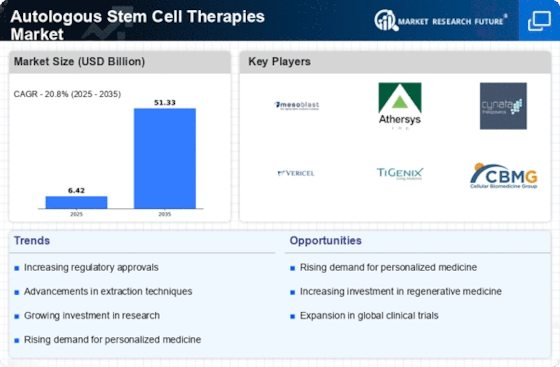
The increasing investment in regenerative medicine is a notable driver for the Autologous Stem Cell Therapies Market. As stakeholders recognize the potential of stem cell therapies to revolutionize treatment paradigms, funding from both public and private sectors has surged. Reports indicate that investments in regenerative medicine reached over USD 10 billion in recent years, reflecting a strong commitment to advancing this field. This influx of capital is likely to accelerate research, enhance clinical trials, and facilitate the commercialization of autologous therapies. Consequently, the Autologous Stem Cell Therapies Market stands to benefit from this trend, as more innovative therapies are developed and brought to market.
Technological Advancements in Stem Cell Research
Technological innovations in stem cell research and therapy development are significantly influencing the Autologous Stem Cell Therapies Market. Advances in cell processing techniques, gene editing technologies, and biomanufacturing processes have enhanced the efficacy and safety of autologous therapies. For instance, the introduction of CRISPR technology has revolutionized the ability to modify stem cells, potentially leading to more effective treatments for various conditions. Furthermore, the market for stem cell therapies is projected to reach USD 20 billion by 2026, driven by these technological advancements. This suggests that ongoing research and development efforts will continue to propel the Autologous Stem Cell Therapies Market forward, fostering innovation and expanding treatment options.
Rising Awareness and Acceptance of Stem Cell Therapies
The growing awareness and acceptance of stem cell therapies among patients and healthcare professionals are driving the Autologous Stem Cell Therapies Market. Educational initiatives and successful case studies have contributed to a more informed public, leading to increased demand for these therapies. Surveys indicate that a significant percentage of patients are willing to consider stem cell treatments for various ailments, reflecting a shift in perception. This heightened awareness is likely to result in more patients seeking autologous therapies, thereby expanding the market. As acceptance continues to grow, the Autologous Stem Cell Therapies Market may witness accelerated adoption and utilization of these innovative treatment options.