Regulatory Support and Frameworks
Regulatory support plays a pivotal role in shaping the Global Autologous Cell Therapy Market Industry. Governments are establishing frameworks that facilitate the approval and commercialization of autologous therapies. These regulations aim to ensure safety and efficacy while promoting innovation. For example, streamlined approval processes for cell-based therapies can expedite their availability to patients. This supportive environment encourages companies to invest in the development of new treatments, thereby driving market growth. As regulatory bodies continue to adapt to the evolving landscape of cell therapies, the industry is likely to experience enhanced momentum.
Rising Prevalence of Chronic Diseases
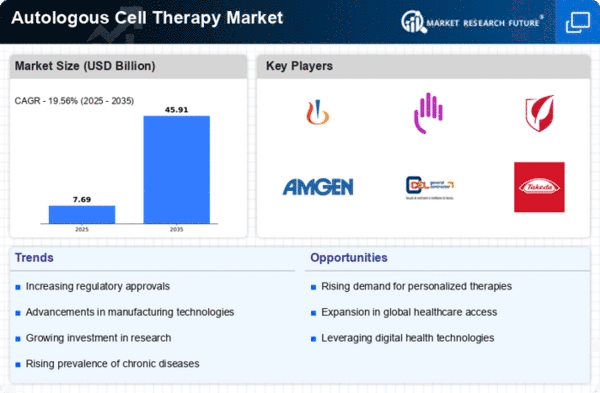
The Global Autologous Cell Therapy Market Industry is experiencing growth due to the increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular disorders. These conditions often require innovative treatment approaches, and autologous cell therapy offers personalized solutions. For instance, the use of stem cells derived from a patient's own body has shown promise in treating various ailments. As the global population ages and the incidence of these diseases rises, the demand for effective therapies is expected to surge. This trend is likely to contribute to the market's projected growth, with an estimated value of 12.8 USD Billion in 2024.
Technological Advancements in Cell Therapy
Technological innovations are significantly shaping the Global Autologous Cell Therapy Market Industry. Advances in cell processing, gene editing, and biomanufacturing techniques enhance the efficacy and safety of therapies. For example, CRISPR technology allows for precise modifications of genetic material, potentially improving treatment outcomes. These advancements not only facilitate the development of new therapies but also streamline production processes, making treatments more accessible. As a result, the market is poised for substantial growth, with projections indicating a rise to 45 USD Billion by 2035, reflecting a compound annual growth rate of 12.13% from 2025 to 2035.
Increased Investment in Research and Development
Investment in research and development is a critical driver of the Global Autologous Cell Therapy Market Industry. Governments and private entities are allocating significant resources to explore the potential of autologous therapies. This funding supports clinical trials and the development of innovative treatment modalities. For instance, various initiatives aim to enhance the understanding of stem cell applications in regenerative medicine. As research progresses, new therapies are likely to emerge, further expanding the market. The anticipated growth trajectory suggests that the industry will continue to attract investment, fostering advancements that could lead to improved patient outcomes.
Growing Awareness and Acceptance of Cell Therapies
The Global Autologous Cell Therapy Market Industry benefits from increasing awareness and acceptance of cell therapies among healthcare professionals and patients. Educational campaigns and successful case studies are helping to demystify these treatments, leading to greater trust and demand. As patients become more informed about the potential benefits of autologous therapies, they are more likely to seek these options. This shift in perception is crucial for market expansion, as it encourages healthcare providers to incorporate these therapies into treatment protocols. Consequently, the market is expected to witness robust growth, driven by a more informed patient population.