Supportive Regulatory Framework
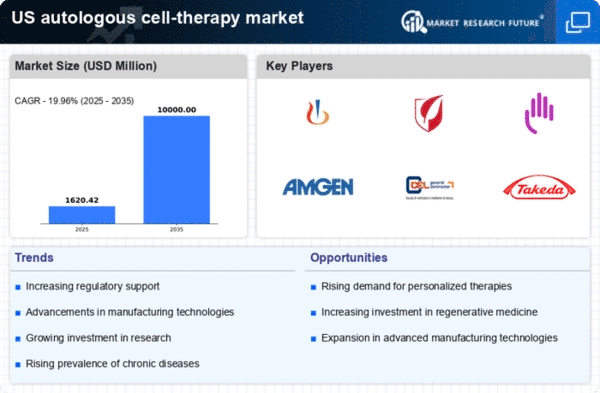
A supportive regulatory framework is essential for the growth of the autologous cell-therapy market. The US Food and Drug Administration (FDA) has established guidelines that facilitate the development and approval of cell therapies, ensuring safety and efficacy. The introduction of the Regenerative Medicine Advanced Therapy (RMAT) designation expedites the review process for promising therapies, encouraging innovation in the field. This regulatory support not only enhances the credibility of autologous therapies but also instills confidence among investors and stakeholders. As more therapies receive regulatory approval, the market is likely to experience accelerated growth, with projections indicating a potential market size of $15 billion by 2030. The proactive stance of regulatory bodies in promoting research and development is a key driver for the autologous cell-therapy market.
Rising Incidence of Chronic Diseases
The increasing prevalence of chronic diseases in the US is a pivotal driver for the autologous cell-therapy market. Conditions such as diabetes, cardiovascular diseases, and cancer are on the rise, necessitating innovative treatment options. According to the Centers for Disease Control and Prevention (CDC), chronic diseases account for 7 out of 10 deaths annually in the US. This alarming statistic underscores the urgent need for effective therapies, including autologous cell therapies, which utilize the patient's own cells to promote healing and recovery. The market is projected to grow as healthcare providers seek to address these chronic conditions with personalized treatment approaches, potentially leading to a market valuation exceeding $10 billion by 2027. As the population ages and the burden of chronic diseases escalates, the demand for autologous cell therapies is likely to intensify.
Advancements in Cell Processing Technologies
Innovations in cell processing technologies are significantly influencing the autologous cell-therapy market. Techniques such as automated cell culture, gene editing, and improved cryopreservation methods enhance the efficiency and efficacy of cell therapies. These advancements facilitate the production of high-quality cells that are essential for successful treatments. For instance, the development of closed-system bioreactors allows for better control over the cell environment, reducing contamination risks and improving yield. As a result, the market is witnessing a surge in the adoption of these technologies, which could lead to a market growth rate of approximately 15% annually. The integration of cutting-edge technologies not only streamlines the manufacturing process but also enhances patient outcomes, thereby driving the overall growth of the autologous cell-therapy market.
Increased Investment in Regenerative Medicine
The surge in investment within the regenerative medicine sector is a crucial driver for the autologous cell-therapy market. Venture capital funding and government grants are increasingly directed towards research and development in this field. In 2024, investments in regenerative medicine reached over $5 billion in the US, reflecting a growing recognition of the potential of autologous therapies. This influx of capital supports clinical trials, product development, and commercialization efforts, thereby accelerating the introduction of innovative therapies to the market. As stakeholders recognize the long-term benefits of regenerative medicine, including cost savings and improved patient outcomes, the autologous cell-therapy market is poised for substantial growth. The financial backing is likely to foster collaborations between academic institutions and industry players, further propelling advancements in this dynamic field.
Growing Awareness and Acceptance of Cell Therapies
The increasing awareness and acceptance of cell therapies among healthcare professionals and patients are driving the autologous cell-therapy market. Educational initiatives and successful case studies are contributing to a more informed public, leading to higher demand for these innovative treatments. As patients become more proactive in seeking personalized medicine options, healthcare providers are responding by integrating autologous therapies into their treatment protocols. Surveys indicate that nearly 60% of patients are willing to consider cell-based therapies for chronic conditions, reflecting a shift in perception. This growing acceptance is likely to enhance market penetration and stimulate further research and development. As the healthcare landscape evolves, the autologous cell-therapy market is expected to benefit from this trend, potentially leading to a market expansion of over 20% in the coming years.