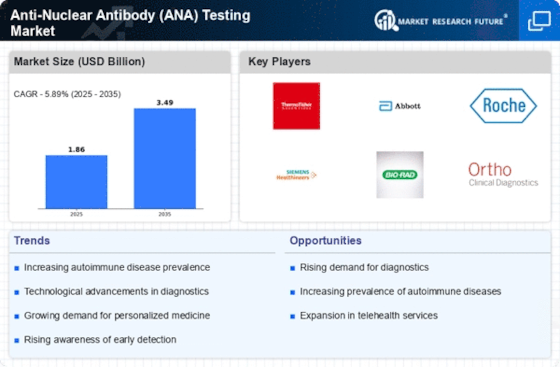
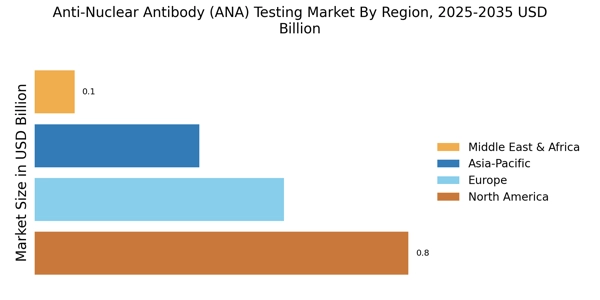
Increased Healthcare Expenditure
Rising healthcare expenditure across various regions is a significant driver for the Anti-Nuclear Antibody (ANA) Testing Market. As governments and private sectors allocate more resources to healthcare, there is a corresponding increase in funding for diagnostic services, including ANA testing. This trend is particularly evident in developed economies, where healthcare spending is projected to grow at an annual rate of approximately 5% over the next few years. Enhanced funding allows for the procurement of advanced testing technologies and the establishment of specialized laboratories, which can cater to the growing demand for ANA tests. Furthermore, increased healthcare expenditure often correlates with improved access to healthcare services, enabling more patients to receive timely and accurate diagnoses. This financial commitment to healthcare infrastructure is likely to bolster the ANA testing market significantly.
Growing Demand for Early Diagnosis
The increasing emphasis on early diagnosis of autoimmune diseases is a crucial driver for the Anti-Nuclear Antibody (ANA) Testing Market. Early detection is vital for effective management and treatment of autoimmune disorders, which can significantly improve patient outcomes. As healthcare systems worldwide prioritize preventive care, the demand for diagnostic tests, including ANA testing, is expected to rise. Studies indicate that early intervention can reduce the long-term healthcare costs associated with chronic autoimmune conditions. Consequently, healthcare providers are more inclined to utilize ANA testing as a proactive measure, thereby expanding its market reach. The trend towards early diagnosis aligns with broader healthcare initiatives aimed at enhancing patient care and reducing the burden of chronic diseases, suggesting a promising trajectory for the ANA testing market.
Rising Awareness and Education Initiatives
The growing awareness of autoimmune diseases and the importance of early diagnosis is a vital driver for the Anti-Nuclear Antibody (ANA) Testing Market. Educational campaigns and initiatives by healthcare organizations are playing a crucial role in informing both healthcare professionals and the public about the symptoms and implications of autoimmune disorders. As awareness increases, more individuals are seeking medical advice and diagnostic testing, including ANA tests. This trend is reflected in the rising number of ANA tests conducted annually, which has seen a steady increase over the past few years. Additionally, healthcare providers are becoming more vigilant in recognizing the signs of autoimmune diseases, leading to higher testing rates. The synergy between awareness initiatives and increased testing demand suggests a robust growth potential for the ANA testing market.
Increasing Prevalence of Autoimmune Diseases
The rising incidence of autoimmune diseases is a pivotal driver for the Anti-Nuclear Antibody (ANA) Testing Market. Conditions such as systemic lupus erythematosus, rheumatoid arthritis, and Sjogren's syndrome are becoming more prevalent, leading to a heightened demand for diagnostic testing. According to recent estimates, autoimmune diseases affect approximately 5-8% of the population, which translates to millions of individuals requiring accurate diagnosis. This growing patient population necessitates the expansion of testing services, thereby propelling the ANA testing market forward. Furthermore, as awareness of these conditions increases, healthcare providers are more likely to recommend ANA testing as a first-line diagnostic tool, further driving market growth. The interplay between rising disease prevalence and increased testing recommendations suggests a robust future for the ANA testing market.
Technological Innovations in Diagnostic Testing
Technological advancements in diagnostic testing methodologies are significantly influencing the Anti-Nuclear Antibody (ANA) Testing Market. Innovations such as enzyme-linked immunosorbent assays (ELISA) and multiplex assays have enhanced the sensitivity and specificity of ANA tests, leading to more accurate diagnoses. The introduction of automated systems and point-of-care testing devices has also streamlined the testing process, reducing turnaround times and improving patient outcomes. As a result, laboratories are increasingly adopting these advanced technologies, which is expected to boost the market. The global market for diagnostic testing is projected to grow at a compound annual growth rate of around 6% over the next few years, indicating a favorable environment for ANA testing advancements. This technological evolution not only enhances diagnostic capabilities but also fosters greater trust in the testing process among healthcare professionals and patients alike.