Growing Geriatric Population
The increasing geriatric population in the United States is a notable driver for the anti nuclear-antibody-testing market. Older adults are more susceptible to autoimmune diseases, which necessitates regular screening and testing. As the population aged 65 and above continues to expand, the prevalence of conditions such as lupus and rheumatoid arthritis is expected to rise. This demographic shift is likely to lead to a higher demand for diagnostic tests, with projections indicating that the market could grow by 10% annually over the next five years. Consequently, the anti nuclear-antibody-testing market must adapt to meet the needs of this aging population, ensuring that testing services are accessible and effective.
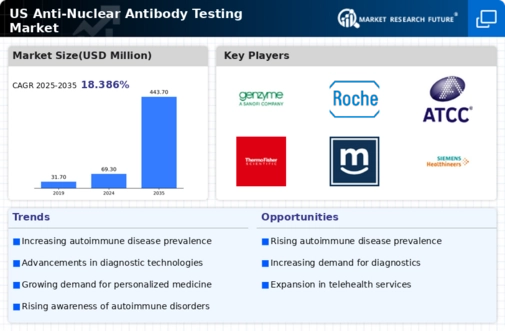
Advancements in Diagnostic Technologies
Technological innovations in diagnostic testing are transforming the anti nuclear-antibody-testing market. The introduction of more sensitive and specific testing methods, such as enzyme-linked immunosorbent assays (ELISA) and multiplex assays, enhances the accuracy of results. These advancements not only improve patient outcomes but also streamline laboratory processes, reducing turnaround times for test results. The market is projected to grow as healthcare facilities adopt these advanced technologies, with a potential increase in market value estimated at $500 million by 2027. As a result, the anti nuclear-antibody-testing market is poised for significant growth, driven by the demand for reliable and efficient diagnostic solutions.
Integration of Telemedicine in Healthcare
The integration of telemedicine into healthcare practices is emerging as a significant driver for the anti nuclear-antibody-testing market. Telehealth services facilitate remote consultations, allowing patients to discuss symptoms and receive referrals for testing without the need for in-person visits. This convenience is particularly beneficial for individuals in rural or underserved areas, where access to healthcare may be limited. As telemedicine continues to gain traction, the demand for at-home testing kits and remote diagnostic services is expected to rise. The anti nuclear-antibody-testing market could see a growth rate of approximately 7% as telehealth becomes a standard practice in managing autoimmune disorders.
Regulatory Support for Diagnostic Testing
Regulatory bodies in the United States are increasingly supporting the development and approval of diagnostic tests, which positively impacts the anti nuclear-antibody-testing market. Initiatives aimed at expediting the approval process for new tests and ensuring their safety and efficacy encourage innovation within the industry. This regulatory environment fosters competition among manufacturers, leading to a wider array of testing options for healthcare providers. As a result, the anti nuclear-antibody-testing market is likely to benefit from enhanced product offerings and improved patient access to necessary diagnostic tools, potentially increasing market growth by 6% over the next few years.
Increasing Awareness of Autoimmune Disorders
The rising awareness of autoimmune disorders among healthcare professionals and patients is a crucial driver for the anti nuclear-antibody-testing market. As more individuals become informed about the symptoms and implications of these conditions, the demand for diagnostic testing increases. Educational campaigns and advocacy groups play a significant role in disseminating information, leading to earlier diagnosis and treatment. This heightened awareness is reflected in the growing number of tests conducted annually, with estimates suggesting a growth rate of approximately 8% in testing frequency over the next few years. Consequently, the anti nuclear-antibody-testing market is likely to experience a corresponding increase in demand as more patients seek testing to confirm or rule out autoimmune diseases.