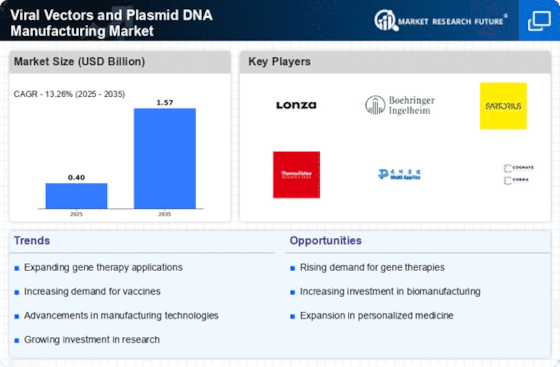
Top Industry Leaders in the Viral Vectors and Plasmid DNA Manufacturing Market

Latest Viral Vectors and Plasmid DNA Manufacturing Companies Update:
Catalent Inaugurated a state-of-the-art plasmid DNA (pDNA) manufacturing facility in Gosselies, Belgium, catering to the rising demand for plasmid DNA and supporting cell and gene therapy companies. (Jan 2023)
BioNTech Completed its in-house clinical and commercial scale plasmid DNA manufacturing facility at Marburg, Germany, strengthening its cell and gene therapy capabilities. (Feb 2023)
Vector Biomed Raised $15 million for its preclinical GMP viral vector manufacturing process, showcasing investor confidence in the market's potential. (Jan 2023)
Sartorius Acquired Polyplus, a plasmid DNA and viral vector production and development company, to enhance their viral vector manufacturing capabilities and cell and gene therapy offerings. (Mar 2023)
Bristol Myers Squibb Announced plans for an in-house viral vector production facility to cater to its cell therapy requirements, indicating vertical integration trends. (Apr 2023)
List of Viral Vectors and Plasmid DNA Manufacturing Key companies in the market
- Cognate BioServices Inc. (US)
- Catalent Pharma Solutions (US)
- Fujifilm Holdings Corporation (Japan)
- Johnson & Johnson (US)
- Sanofi Corporation (France)
- Hoffmann-LA Roche Ltd (Switzerland)
- 4D Molecular Therapeutics (US)
- Sirion Biotech GmbH (Germany)
- Voyager Therapeutics (US)
- Thermo Fisher Scientific Inc. (US)
- Gene Therapy Catapult (UK)
- UniQure (Netherlands)
- MassBiologics (US)
- Renova Therapeutics (US)
- Shenzhen SiBionoGeneTech Co. Ltd (China)