US Sterility Testing Market Summary
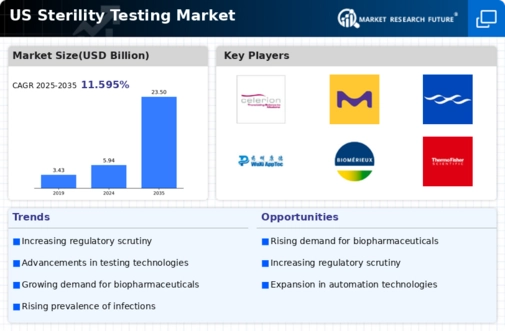
The US Sterility Testing Market is projected to grow significantly from 5.94 USD Billion in 2024 to 23.5 USD Billion by 2035.
Key Market Trends & Highlights
US Sterility Testing Market Key Trends and Highlights
- The market is expected to experience a compound annual growth rate (CAGR) of 13.32% from 2025 to 2035.
- By 2035, the market valuation is anticipated to reach 23.5 USD Billion, indicating robust growth.
- In 2024, the market is valued at 5.94 USD Billion, reflecting a strong foundation for future expansion.
- Growing adoption of advanced sterilization technologies due to increasing regulatory requirements is a major market driver.
Market Size & Forecast
| 2024 Market Size | 5.94 (USD Billion) |
| 2035 Market Size | 23.5 (USD Billion) |
| CAGR (2025-2035) | 13.32% |
Major Players
Celerion, Sterigenics International, Abbott Laboratories, Roka Bioscience, Merck KGaA, Pace Analytical Services, Promega Corporation, Charles River Laboratories, MilliporeSigma, SGS SA, Becton Dickinson, Wuxi Apptec, BioMerieux, Thermo Fisher Scientific, Essen BioScience