Expansion of Emerging Markets
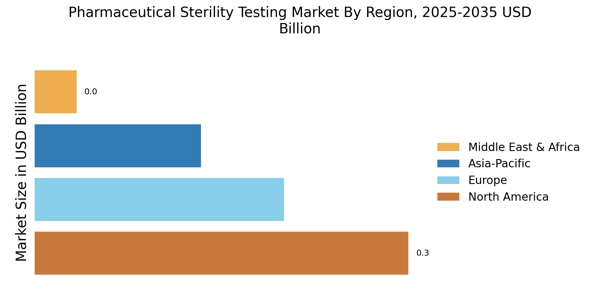
The Pharmaceutical Sterility Testing Market Industry is witnessing growth driven by the expansion of emerging markets. As economies in regions such as Asia-Pacific and Latin America develop, there is an increasing demand for pharmaceutical products, which in turn necessitates robust sterility testing protocols. Emerging markets are experiencing a rise in pharmaceutical manufacturing capabilities, leading to a greater emphasis on quality assurance and compliance with international standards. This trend is likely to create new opportunities for sterility testing service providers as they cater to the evolving needs of these markets. The Pharmaceutical Sterility Testing Market Industry stands to benefit from this expansion, as companies seek to establish a foothold in these burgeoning regions.
Growing Awareness of Patient Safety
The Pharmaceutical Sterility Testing Market Industry is significantly influenced by the increasing awareness surrounding patient safety. As healthcare providers and patients become more informed about the risks associated with contaminated pharmaceuticals, the demand for rigorous sterility testing intensifies. This heightened awareness is prompting pharmaceutical companies to prioritize sterility assurance in their product development processes. Furthermore, the rise of social media and information dissemination platforms has amplified discussions about product safety, leading to greater scrutiny of pharmaceutical practices. Consequently, companies are compelled to invest in comprehensive sterility testing to uphold their reputations and ensure consumer trust. This trend is likely to bolster the Pharmaceutical Sterility Testing Market Industry as stakeholders recognize the critical importance of sterility in safeguarding public health.
Regulatory Compliance and Standards
The Pharmaceutical Sterility Testing Market Industry is heavily influenced by stringent regulatory requirements imposed by health authorities. Regulatory bodies such as the FDA and EMA mandate rigorous sterility testing protocols to ensure the safety and efficacy of pharmaceutical products. Compliance with these regulations is not merely a formality; it is a critical component of product development and market entry. As a result, pharmaceutical companies are increasingly investing in advanced sterility testing methods to meet these standards. The market for sterility testing is projected to grow as companies seek to avoid costly penalties and product recalls associated with non-compliance. This trend indicates a robust demand for reliable sterility testing solutions, thereby driving growth in the Pharmaceutical Sterility Testing Market Industry.
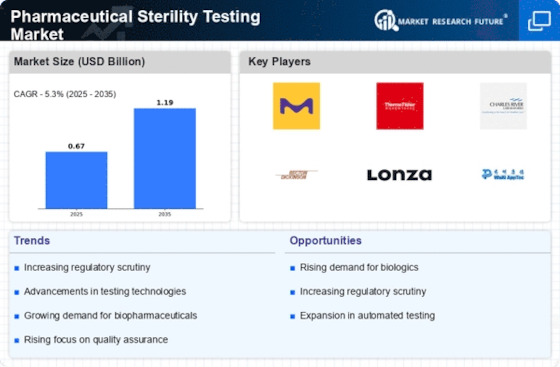
Rising Demand for Biopharmaceuticals
The Pharmaceutical Sterility Testing Market Industry is experiencing a surge in demand due to the increasing prevalence of biopharmaceuticals. As the biopharmaceutical sector expands, the need for effective sterility testing becomes paramount. Biopharmaceuticals, which include monoclonal antibodies and vaccines, require stringent sterility assurance to prevent contamination and ensure patient safety. According to industry reports, the biopharmaceutical market is expected to reach substantial figures in the coming years, thereby propelling the demand for sterility testing services. This growth is likely to create opportunities for companies specializing in sterility testing, as they adapt their offerings to meet the unique challenges posed by biopharmaceutical products. Consequently, the Pharmaceutical Sterility Testing Market Industry is poised for significant expansion.
Technological Innovations in Testing Equipment
Technological advancements play a pivotal role in shaping the Pharmaceutical Sterility Testing Market Industry. Innovations in testing equipment, such as automated systems and rapid testing methods, enhance the efficiency and accuracy of sterility testing processes. These advancements not only reduce testing times but also minimize human error, which is crucial in maintaining the integrity of pharmaceutical products. The introduction of novel technologies, such as real-time PCR and next-generation sequencing, is expected to revolutionize sterility testing protocols. As pharmaceutical companies strive for faster time-to-market, the demand for cutting-edge testing solutions is likely to increase. This trend suggests a dynamic shift in the Pharmaceutical Sterility Testing Market Industry, driven by the need for improved testing methodologies.