US Safety Lancet Market Summary
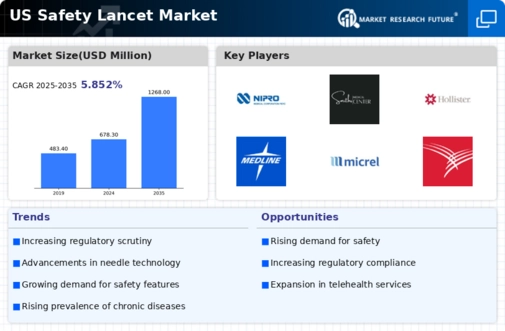
The US Safety Lancet Market is projected to grow from 678.3 million USD in 2024 to 1268 million USD by 2035.
Key Market Trends & Highlights
US Safety Lancet Market Key Trends and Highlights
- The market is expected to experience a compound annual growth rate (CAGR) of 5.85 percent from 2025 to 2035.
- By 2035, the market valuation is anticipated to reach 1268 million USD, indicating robust growth potential.
- In 2024, the market is valued at 678.3 million USD, reflecting a solid foundation for future expansion.
- Growing adoption of safety technologies due to increasing regulatory requirements is a major market driver.
Market Size & Forecast
| 2024 Market Size | 678.3 (USD Million) |
| 2035 Market Size | 1268 (USD Million) |
| CAGR (2025-2035) | 5.85% |
Major Players
Nipro Medical Corporation, Smiths Medical, Hollister Incorporated, Medline Industries, HMD Medical, Becton Dickinson and Company, Micrel Medical Devices, Alphatec Spine, Terumo Corporation, Wrigley Medical, GrahamField Health Products, Cardinal Health