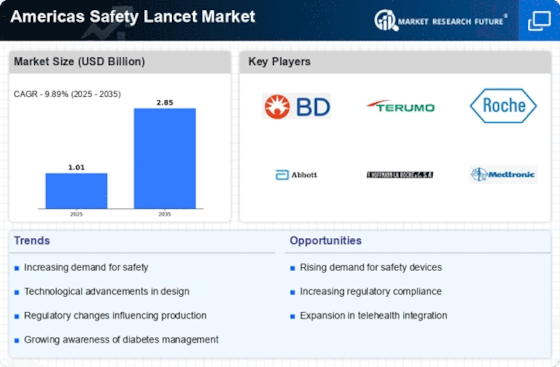
The Americas Safety Lancet Market is characterized by a dynamic competitive landscape, driven by a confluence of innovation, regulatory compliance, and an increasing emphasis on patient safety. Key players such as BD (US), Terumo Corporation (JP), and Abbott Laboratories (US) are at the forefront, each adopting distinct strategies to enhance their market positioning. BD (US) has focused on expanding its product portfolio through innovation, particularly in safety-engineered devices, which aligns with the growing demand for safer medical practices. Terumo Corporation (JP) emphasizes regional expansion and strategic partnerships, enhancing its distribution networks across North America. Meanwhile, Abbott Laboratories (US) is leveraging digital transformation to improve patient outcomes and streamline operations, indicating a shift towards technology-driven healthcare solutions. Collectively, these strategies contribute to a competitive environment that is increasingly focused on safety, efficiency, and technological advancement.In terms of business tactics, companies are localizing manufacturing and optimizing supply chains to enhance responsiveness to market demands. The competitive structure of the Americas Safety Lancet Market appears moderately fragmented, with several key players exerting influence while also facing competition from smaller, specialized firms. This fragmentation allows for diverse product offerings and innovation, as companies strive to differentiate themselves in a crowded marketplace.
In August BD (US) announced the launch of a new safety lancet designed to minimize the risk of needlestick injuries, a move that underscores its commitment to patient safety and innovation. This strategic action not only enhances BD's product portfolio but also positions the company as a leader in addressing critical healthcare challenges. The introduction of this device is likely to resonate well with healthcare providers seeking to improve safety protocols in clinical settings.
In September Terumo Corporation (JP) entered into a strategic partnership with a leading telehealth provider to integrate its safety lancet products into remote patient monitoring systems. This collaboration is indicative of a broader trend towards digital health solutions, suggesting that Terumo is keen on leveraging technology to enhance patient care and expand its market reach. Such partnerships may facilitate the adoption of safety lancets in telehealth applications, thereby broadening their utility and market penetration.
In October Abbott Laboratories (US) unveiled a new digital platform aimed at healthcare professionals, providing real-time data analytics on the use of safety lancets. This initiative reflects Abbott's focus on integrating artificial intelligence and data analytics into its product offerings, potentially transforming how healthcare providers manage patient safety. By equipping professionals with actionable insights, Abbott is likely to enhance the value proposition of its safety lancet products, fostering greater adoption in clinical environments.
As of October the competitive trends in the Americas Safety Lancet Market are increasingly defined by digitalization, sustainability, and the integration of artificial intelligence. Strategic alliances are becoming pivotal, as companies recognize the need to collaborate to enhance their technological capabilities and market reach. Looking ahead, competitive differentiation is expected to evolve, with a pronounced shift from price-based competition to a focus on innovation, technological advancements, and supply chain reliability. This transition may ultimately redefine the parameters of success in the market, as companies strive to meet the evolving needs of healthcare providers and patients.