Regulatory Incentives for Biologics
Regulatory incentives provided by the US government are playing a pivotal role in the growth of the pegylated drugs market. Initiatives such as the Orphan Drug Act and the Biologics Control Act encourage the development of biologics, including pegylated drugs, by offering benefits like tax credits and extended market exclusivity. These incentives are particularly attractive for companies developing treatments for rare diseases, which often utilize pegylation to enhance drug properties. As a result, the number of pegylated drugs entering the market is expected to increase, potentially leading to a 20% rise in market size by 2027, as more companies take advantage of these regulatory benefits.
Advancements in Drug Delivery Systems
Innovations in drug delivery systems are significantly impacting the pegylated drugs market. The development of advanced formulations that enhance the bioavailability and stability of pegylated drugs is crucial. These advancements allow for more efficient delivery of therapeutics, leading to improved patient outcomes. For example, pegylated liposomal formulations are being utilized to enhance the delivery of chemotherapeutic agents, thereby increasing their effectiveness while reducing side effects. The US market is witnessing a shift towards these advanced delivery systems, which could potentially increase the market share of pegylated drugs by 15% over the next few years, as healthcare providers seek more effective treatment modalities.
Rising Prevalence of Chronic Diseases
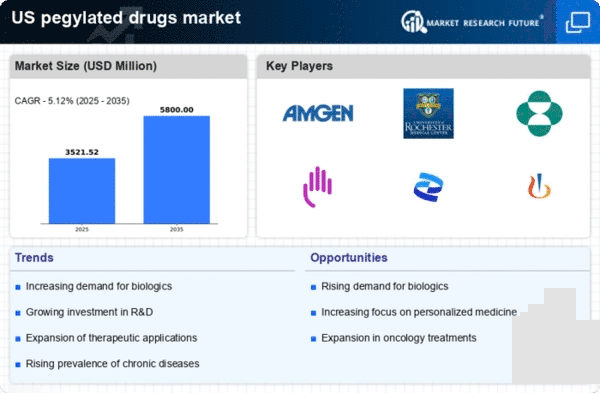
The pegylated drugs market is experiencing growth due to the increasing prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders in the US. As these conditions become more common, the demand for effective treatment options rises. Pegylated drugs, known for their extended half-life and improved therapeutic efficacy, are becoming essential in managing these diseases. For instance, pegylated interferon is widely used in the treatment of hepatitis C, which affects millions of Americans. The market for pegylated drugs is projected to reach approximately $20 billion by 2026, indicating a robust growth trajectory driven by the need for innovative therapies in chronic disease management.
Increased Focus on Patient-Centric Approaches
The pegylated drugs market is increasingly influenced by a shift towards patient-centric approaches in healthcare. This trend emphasizes the importance of tailoring treatments to individual patient needs, which aligns well with the characteristics of pegylated drugs. These therapies often provide improved dosing regimens and reduced side effects, enhancing patient compliance and satisfaction. As healthcare providers and pharmaceutical companies prioritize patient outcomes, the demand for pegylated drugs is likely to grow. Market analysts predict that this focus on patient-centricity could contribute to a 10% increase in the pegylated drugs market by 2026, as more patients seek effective and convenient treatment options.
Growing Investment in Biopharmaceutical Research
The pegylated drugs market is benefiting from increased investment in biopharmaceutical research and development. Pharmaceutical companies are allocating substantial resources to discover and develop new pegylated therapies, driven by the potential for high returns on investment. In 2025, the US biopharmaceutical sector is expected to invest over $100 billion in R&D, with a significant portion directed towards pegylated drug development. This influx of funding is likely to accelerate the introduction of novel pegylated therapies, thereby expanding the market. The focus on innovative treatments aligns with the industry's goal of addressing unmet medical needs, further propelling the growth of the pegylated drugs market.