Regulatory Support for Biologics
The regulatory environment in Japan is becoming increasingly supportive of biologics, including pegylated drugs. The Pharmaceuticals and Medical Devices Agency (PMDA) has streamlined approval processes for innovative therapies, encouraging the development of new pegylated formulations. This regulatory support is crucial, as it reduces the time and cost associated with bringing new drugs to market. As a result, pharmaceutical companies are more inclined to invest in the development of pegylated drugs, knowing that there is a favorable regulatory framework in place. The pegylated drugs market is likely to experience growth as a direct consequence of this supportive regulatory landscape, facilitating the introduction of novel therapies.
Advancements in Drug Delivery Systems
Innovations in drug delivery systems are transforming the landscape of the pegylated drugs market. The development of novel formulations and delivery mechanisms enhances the efficacy and safety of pegylated therapies. For instance, the integration of nanotechnology and targeted delivery systems allows for more precise administration of drugs, minimizing side effects and improving therapeutic outcomes. In Japan, the focus on personalized medicine is driving research and development in this area, with investments in advanced drug delivery technologies. The pegylated drugs market is likely to benefit from these advancements, as they enable the creation of more effective and patient-friendly treatment options.
Increasing Prevalence of Chronic Diseases
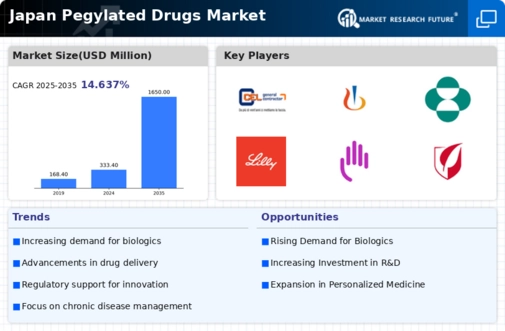
The rising incidence of chronic diseases in Japan is a pivotal driver for the pegylated drugs market. Conditions such as cancer, diabetes, and autoimmune disorders are becoming more prevalent, necessitating advanced therapeutic options. According to recent health statistics, chronic diseases account for approximately 60% of all deaths in Japan, highlighting the urgent need for effective treatments. Pegylated drugs, known for their extended half-life and reduced immunogenicity, are increasingly being utilized to manage these conditions. This trend is likely to continue, as healthcare providers seek innovative solutions to improve patient outcomes. The pegylated drugs market is thus positioned to grow significantly, driven by the demand for therapies that can effectively address the complexities of chronic disease management.
Rising Awareness of Personalized Medicine
The increasing awareness and adoption of personalized medicine in Japan is driving demand for pegylated drugs. Healthcare providers and patients are recognizing the benefits of tailored therapies that consider individual patient characteristics, leading to improved treatment outcomes. Pegylated drugs, with their ability to enhance drug efficacy and reduce side effects, align well with the principles of personalized medicine. As more healthcare professionals advocate for personalized treatment approaches, the pegylated drugs market is expected to expand. This shift towards personalized medicine is likely to influence research priorities and investment strategies, further propelling the development of pegylated therapies.
Growing Investment in Biopharmaceutical Research
The surge in investment in biopharmaceutical research is a significant catalyst for the pegylated drugs market. Japan has established itself as a hub for biopharmaceutical innovation, with government initiatives and private sector funding supporting research and development. In recent years, the biopharmaceutical sector has seen an increase in funding, with estimates suggesting a growth rate of over 10% annually. This influx of capital is facilitating the exploration of pegylated drug formulations, which are often more complex and require substantial investment in research. The pegylated drugs market stands to gain from this trend, as increased funding leads to the discovery of new therapies and improved treatment options.





















Leave a Comment