Technological Advancements in Medical Devices
Technological advancements are reshaping the medical devices-reimbursement market, as innovations such as telemedicine, wearable devices, and minimally invasive surgical tools gain traction. These technologies not only enhance patient care but also improve operational efficiencies within healthcare systems. For instance, the integration of artificial intelligence in diagnostic devices is expected to streamline processes and reduce costs. As these technologies become more prevalent, reimbursement frameworks are likely to evolve to include coverage for new modalities. The medical devices-reimbursement market may see a shift in payer policies to reflect the value these innovations bring to patient outcomes and healthcare delivery.
Aging Population and Chronic Disease Prevalence
The demographic shift towards an aging population in the United States is significantly impacting the medical devices-reimbursement market. As individuals age, the prevalence of chronic diseases such as diabetes, cardiovascular conditions, and arthritis increases. This demographic trend necessitates the use of various medical devices for monitoring and treatment. It is estimated that by 2030, nearly 20% of the U.S. population will be over 65 years old, leading to heightened demand for medical devices. Payers are likely to adapt their reimbursement strategies to accommodate the growing need for these devices, ensuring that patients have access to necessary treatments. This evolving landscape presents opportunities for manufacturers to align their offerings with reimbursement policies.
Focus on Cost-Effectiveness and Health Economics
Cost-effectiveness is becoming a critical consideration in the medical devices-reimbursement market. Payers are increasingly evaluating the economic impact of devices, seeking to ensure that expenditures align with patient outcomes. This focus on health economics may lead to more rigorous assessments of the value provided by medical devices. Manufacturers are encouraged to present robust data demonstrating the cost-effectiveness of their products to secure favorable reimbursement terms. As a result, the medical devices-reimbursement market may witness a shift towards evidence-based reimbursement models that prioritize both clinical efficacy and economic viability.
Increasing Demand for Innovative Medical Devices
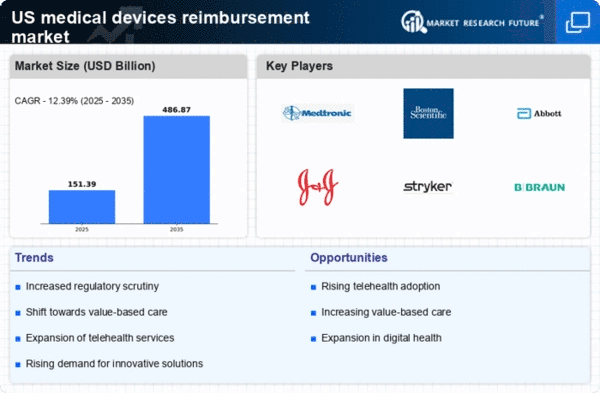
The medical devices-reimbursement market is experiencing a notable surge in demand for innovative medical devices. This trend is driven by advancements in technology and an increasing focus on patient outcomes. As healthcare providers seek to improve treatment efficacy, the adoption of cutting-edge devices is likely to rise. According to recent data, the market for innovative medical devices is projected to grow at a CAGR of approximately 7.5% over the next five years. This growth is expected to influence reimbursement policies, as payers are more inclined to cover devices that demonstrate clear clinical benefits. Consequently, manufacturers are incentivized to invest in research and development, further propelling the medical devices-reimbursement market forward.
Regulatory Environment and Reimbursement Policies
The regulatory environment surrounding the medical devices-reimbursement market is complex and continually evolving. Recent changes in policies by the Centers for Medicare & Medicaid Services (CMS) have implications for how devices are reimbursed. For example, the introduction of new coding systems and reimbursement models can affect the financial viability of certain devices. Manufacturers must navigate these regulations to ensure compliance and optimize reimbursement rates. The medical devices-reimbursement market is likely to be influenced by ongoing discussions regarding the balance between innovation and regulatory oversight, as stakeholders seek to ensure patient safety while promoting access to advanced medical technologies.